Hydrogen Peroxide
Category : JEE Main & Advanced
Hydrogen peroxide \[({{H}_{2}}{{O}_{2}})\] was discovered by French chemist Thenard.
(1) Preparation : It is prepared by
(i) Laboratory method : In laboratory, \[{{H}_{2}}{{O}_{2}}\] is prepared by Merck's process. It is prepared by adding calculated amounts of sodium peroxide to ice cold dilute (20%) solution of \[{{H}_{2}}S{{O}_{4}}\].
\[N{{a}_{2}}{{O}_{2}}+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
(ii) By the action of sulphuric acid or phosphoric acid on hydrated barium peroxide \[Ba{{O}_{2}}.8{{H}_{2}}O\]
(a) \[Ba{{O}_{2}}.8{{H}_{2}}O+{{H}_{2}}S{{O}_{4}}\to BaS{{O}_{4}}\downarrow +{{H}_{2}}{{O}_{2}}+8{{H}_{2}}O\]
It must be noted that anhydrous barium peroxide does not react readily with sulphuric acid (because a coating of insoluble barium sulphate is formed on its surface which stops further action of the acid). Therefore, hydrated barium peroxide, \[Ba{{O}_{2}}.8{{H}_{2}}O\] must be used.
(b) \[3Ba{{O}_{2}}+2{{H}_{3}}P{{O}_{4}}\to B{{a}_{3}}{{(P{{O}_{4}})}_{2}}+3{{H}_{2}}{{O}_{2}}\]
\[B{{a}_{3}}{{(P{{O}_{4}})}_{2}}+3{{H}_{2}}S{{O}_{4}}\to 3BaS{{O}_{4}}+2{{H}_{3}}P{{O}_{4}}\]
Phosphoric acid is preferred to \[{{H}_{2}}S{{O}_{4}}\] because soluble impurities like barium persulphate (from \[Ba{{O}_{2}}.8{{H}_{2}}O+{{H}_{2}}S{{O}_{4}}\]) tends to decompose \[{{H}_{2}}{{O}_{2}}\] while \[{{H}_{3}}P{{O}_{4}}\] acts as preservative (negative catalyst) for \[{{H}_{2}}{{O}_{2}}\].
(iii) Industrial method : On a commercial scale, \[{{H}_{2}}{{O}_{2}}\] can be prepared by the electrolysis of 50% \[{{H}_{2}}S{{O}_{4}}\] solution. In a cell, peroxy disulphuric acid is formed at the anode.
\[2{{H}_{2}}S{{O}_{4}}\xrightarrow[\text{Elecrolysis}]{}\underset{\begin{smallmatrix} \text{Peroxy disulphuric} \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{acid} \end{smallmatrix}}{\mathop{{{H}_{2}}{{S}_{2}}{{O}_{8}}(aq.)}}\,+{{H}_{2}}(g)\]
This is drawn off from the cell and hydrolysed with water to give \[{{H}_{2}}{{O}_{2}}\].
\[{{H}_{2}}{{S}_{2}}{{O}_{8}}+2{{H}_{2}}O\xrightarrow{{}}2{{H}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\]
The resulting solution is distilled under reduced pressure when \[{{H}_{2}}{{O}_{2}}\] gets distilled while \[{{H}_{2}}S{{O}_{4}}\] with high boiling point, remains undistilled.
(iv) By redox process : Industrially \[{{H}_{2}}{{O}_{2}}\] is prepared by the auto-oxidation of 2-alkylanthraquinols. The process involves a cycle of reactions. The net reaction is the catalytic union of \[{{H}_{2}}\] and \[{{O}_{2}}\] to give \[{{H}_{2}}{{O}_{2}}\].
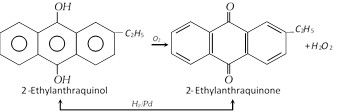
The \[{{H}_{2}}{{O}_{2}}\] formed (about 1%) is extracted with water and concentrated.
(2) Physical properties
(i) Pure hydrogen peroxide is a pale blue syrupy liquid.
(ii) It freezes at - 0.5°C and has a density of 1.4 in pure state.
(iii) Hydrogen peroxide is diamagnetic.
(iv) It is more highly associated via hydrogen bonding than water.
(v) Although it is a better polar solvent than \[{{H}_{2}}O\].
However, it can?t be used as such because of strong autooxidation ability.
(vi) Dipole moment of \[{{H}_{2}}{{O}_{2}}\] is 2.1 D.
(3) Chemical properties
(i) Decomposition : Pure \[{{H}_{2}}{{O}_{2}}\] is an unstable liquid and decomposes into water and \[{{O}_{2}}\] either upon standing or upon heating, \[2{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}};\,\,\,\Delta H=-196.0\,kJ\]
(ii) Oxidising nature : It is a powerful oxidising agent. It acts as an oxidising agent in neutral, acidic or in alkaline medium. e.g. \[2KI+{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2KOH+{{I}_{2}}\] [In neutral medium]
\[2FeS{{O}_{4}}+{{H}_{2}}S{{O}_{4}}+{{H}_{2}}{{O}_{2}}\xrightarrow{{}}F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+2{{H}_{2}}O\] [In acidic medium]
\[MnS{{O}_{4}}+{{H}_{2}}{{O}_{2}}+2NaOH\xrightarrow{{}}Mn{{O}_{2}}+N{{a}_{2}}S{{O}_{4}}+2{{H}_{2}}O\]
[In alkaline medium]
(iii) Reducing nature : \[{{H}_{2}}{{O}_{2}}\] has tendency to take up oxygen from strong oxidising agents and thus, acts as a reducing agent, \[\underset{\begin{smallmatrix} \text{From oxidising } \\ \,\,\,\,\,\,\,\,\,\,\text{agent} \end{smallmatrix}}{\mathop{{{H}_{2}}{{O}_{2}}+O\xrightarrow{{}}{{H}_{2}}O+{{O}_{2}}}}\,\]. It can act as a reducing agent in acidic, basic or even neutral medium.
In acidic medium, \[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}2{{H}^{+}}+{{O}_{2}}+2{{e}^{-}}\]
In alkaline medium, \[{{H}_{2}}{{O}_{2}}+2O{{H}^{-}}\xrightarrow{{}}2{{H}_{2}}O+{{O}_{2}}+2{{e}^{-}}\]
(iv) Bleaching action : \[{{H}_{2}}{{O}_{2}}\] acts as a bleaching agent due to the release of nascent oxygen.
\[{{H}_{2}}{{O}_{2}}\xrightarrow{{}}{{H}_{2}}O+O\]
Thus, the bleaching action of \[{{H}_{2}}{{O}_{2}}\] is due to oxidation. It oxidises the colouring matter to a colourless product, Colouring matter + O \[\to \] Colour less matter.
\[{{H}_{2}}{{O}_{2}}\] is used to bleach delicate materials like ivory, silk, wool, leather etc.
(v) Acidic nature : Anhydrous hydrogen peroxide is acidic in character \[({{K}_{a}}=1.55\times {{10}^{-12}}\] at 298 K). its dissociation in aqueous solution may be given as
\[{{H}_{2}}{{O}_{2}}+{{H}_{2}}O\to {{H}_{3}}{{O}^{+}}+HO_{2}^{-}\]
It forms two types of salts
\[NaOH+{{H}_{2}}{{O}_{2}}\to \underset{\begin{smallmatrix} \text{Sod}\text{. hydroperoxide} \\ \text{ (Acidic salt)} \end{smallmatrix}}{\mathop{NaH{{O}_{2}}}}\,+\ \ {{H}_{2}}O\]
\[2NaOH+{{H}_{2}}{{O}_{2}}\to \underset{\begin{smallmatrix} \text{Sod}\text{. peroxide} \\ \text{ (Normal salt)} \end{smallmatrix}}{\mathop{N{{a}_{2}}{{O}_{2}}}}\,+\ \ 2{{H}_{2}}O\]
(vi) Addition reactions : Hydrogen peroxide is capable of adding itself to ethylenic linkage.
\[\underset{\text{Ethylene}}{\mathop{\underset{C{{H}_{2}}}{\overset{C{{H}_{2}}}{\mathop{||\,\,\ \ \ }}}\,}}\,+\ {{H}_{2}}{{O}_{2}}\ \to \ \underset{\text{Ethylene glycol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{|\ \ \ \ \ \ \ \ \ \ \ }}}\,}}\,\]
(4) Structure of H2O2 : Hydrogen peroxide is non-linear, non-planar molecule. It has a open book structure. The \[-O-O-\] linkage is called peroxy linkage. The structure is shown below.
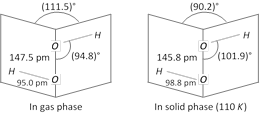
(5) Concentration of H2O2 : Dilute \[{{H}_{2}}{{O}_{2}}\] is concentrated to about 50% by slow evaporation on a water bath. It is further concentrated to 90% in a vacuum desiccator using conc.\[{{H}_{2}}S{{O}_{4}}\] as dehydrating agent. Further concentration to 99% is obtained by distillation under reduced pressure. Last traces of moisture in 99% of \[{{H}_{2}}{{O}_{2}}\] are removed or anhydrous \[{{H}_{2}}{{O}_{2}}\] is obtained by cooling it to 263 K in a cold bath of ether and dry ice followed by seeding with a few crystals of solid \[{{H}_{2}}{{O}_{2}}\] when needle-shaped crystals of 100% \[{{H}_{2}}{{O}_{2}}\] separate out. These crystals are removed, dried and melted to get 100% \[{{H}_{2}}{{O}_{2}}\]. (6) Storage of H2O2 : \[{{H}_{2}}{{O}_{2}}\] is not stored in glass bottles since the alkali metal oxides present in glass catalyse its decomposition. It is, therefore, stored in paraffin wax coated glass, plastic or teflon bottles. Small amounts of acid, glycerol, alcohol, acetanilide and \[{{H}_{3}}P{{O}_{4}}\] are often used as stablizers to check its decomposition.
Uses of hydrogen peroxide
(i) For bleaching delicate articles like wool, hair, feather, ivory, etc.
(ii) For restoring colour of old lead paintings whose white lead has blackened due to formation of PbS by \[{{H}_{2}}S\] of atmosphere. Hydrogen peroxide converts the black lead sulphide to white lead sulphate
(iii) As an aerating agent in production of spong rubber. (iv) As an antiseptic and germicide for washing wounds, teeth and ears, under the name of perhydrol. (v) In the manufacture of sodium perborate, sodium percarbonate. These are used in high quality detergents.
(vi) As an antichlor.
(vii) As an oxidant for rocket fuel.
(viii) In the detection of Ti, V and Cr ions with which it forms peroxides of characteristics colours.
(ix) In the production of epoxides, propylene oxide and polyurethanes.
(x) In the synthesis of hydroquinone, pharmaceuticals (cephalosoporin) and food products like tartaric acid.
(xi) For pollution control of domestic effluents where it restores the aerobic conditions of sewage wastes. For pollution control of industrial effluents containing \[C{{N}^{-}}\] ions. \[{{H}_{2}}{{O}_{2}}\] oxidises \[C{{N}^{-}}\] ions to harmless products.
You need to login to perform this action.
You will be redirected in
3 sec
