Directive Effect In Substituted Benzene Derivatives
Category : JEE Main & Advanced
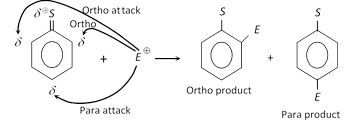
(1) Directive effect in mono substituted benzene derivatives : The substituent already present on the benzene ring directs the incoming substituent to occupy ortho (2 or 6), meta (3 or 5) or para (4) position. This direction depends on the nature of the first substituent and is called directive or the orientation effect.
The substituent already present can increase or decrease the rate of further substitution, i.e., it either activates or deactivates the benzene ring towards further substitution. These effects are called activity effects.
There are two types of substituents which produce directive effect are,
(i) Those which direct the incoming group to ortho- and para-positions simultaneously (Neglecting meta all together).
(ii) Those which direct the incoming group to meta-position only (Neglecting ortho- and para-positions all together).
| Ortho-para directors | Meta directors |
|
Strongly activating \[-\overset{.\,.\,\,\,\,\,}{\mathop{N{{H}_{2}}}}\,,-\overset{.\,.\,\,\,\,\,}{\mathop{NHR}}\,,-\overset{.\,.\,\,\,\,\,}{\mathop{N{{R}_{2}}}}\,,\]
|
Moderately deactivating \[-C\equiv N,-S{{O}_{3}}H,\] \[-COOH,-COOR,\] \[-CHO,COR\] |
|
Moderately activating \[\overset{.\ .\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-NHCOC{{H}_{3}}}}\,,\,\overset{.\ .\,\,\,\,\,\,\,\,\,\,}{\mathop{-NHCOR}}\,,\] |
Strongly deactivating \[-N{{O}_{2}},-N{{R}_{3}}^{\oplus },-C{{F}_{3}},-CC{{l}_{3}}\] |
|
Weakly activating \[-C{{H}_{3}},-{{C}_{2}}{{H}_{5}},-R,-{{C}_{6}}{{H}_{5}}\] Weakly deactivating |
Theory of ortho - para directing group
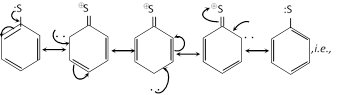
The above mechanism is followed when S is \[-OH,\,-N{{H}_{2}},-Cl,\,-Br,-I,-OR,-N{{R}_{2}},-NHCOR\] etc.
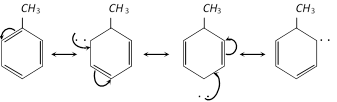
In methyl or alkyl group, the +I effect of the methyl group or alkyl group initiates the resonance effect.
Thus, methyl or alkyl group directs all electrophiles to ortho and para positions.
Theory of meta directing group : The substituent, S withdraws electrons from ortho and para positions. Thus, m-position becomes a point of relatively high electron density and further substitution by electrophile occurs at meta position. For example, \[-N{{O}_{2}}\] group is a meta directing (Electron withdrawing). Its mechanism can be explained as :
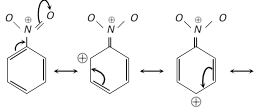
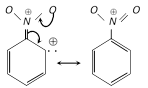
All meta-directing groups have either a partial positive charge or a full positive charge on the atom directly attached to the ring.
(2) Directive effect in disubstituted benzene
(i) If the directive effects of two substituents reinforce, then a single product is formed.
Example :
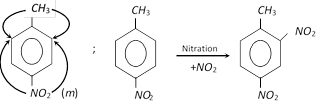
Thus, both \[(C{{H}_{3}},N{{O}_{2}})\] direct further substitution to the same position (Ortho with respect to \[C{{H}_{3}}\]).
(ii) If the directing effect of two groups oppose each other strongly activating groups win over deactivating or weakly activating group. The sequence of directing power is
\[-N{{H}_{2}}>-OH>-OC{{H}_{3}}->NHCOC{{H}_{3}}>-{{C}_{6}}{{H}_{5}}>C{{H}_{3}}>\] meta directors
Example :
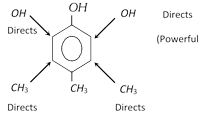
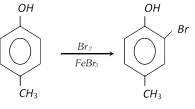
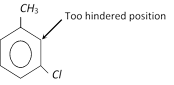
(iii) There is normally little substitution when the two groups are meta to each other. Aromatic rings with three adjacent substituents are generally prepared by same other routes.
You need to login to perform this action.
You will be redirected in
3 sec
