Hyperconjugative Effect
Category : JEE Main & Advanced
(1) When a \[H-C\] bond is attached to an unsaturated system such as double bond or a benzene ring, the sigma \[(\sigma )\] electrons of the \[H-C\] bond interact or enter into conjugation with the unsaturated system. The interactions between the electrons of \[\pi \] systems (multiple bonds) and the adjacent \[\sigma \] bonds (single \[H-C\] bonds) of the substituent groups in organic compounds is called hyperconjugation. The concept of hyperconjugation was developed by Baker and Nathan and is also known as Baker and Nathan effect.
In fact hyperconjugation effect is similar to resonance effect. Since there is no bond between the \[\alpha \]-carbon atom and one of the hydrogen atoms, the hyperconjugation is also called no-bond resonance.
(2) Structural requirements for hyperconjugation
(i) Compound should have at least one \[s{{p}^{2}}\]-hybrid carbon of either alkene alkyl carbocation or alkyl free radical.
(ii) \[\alpha \]-carbon with respect to \[s{{p}^{2}}\]hybrid carbon should have at least one hydrogen.
If both these conditions are fulfilled then hyperconjugation will take place in the molecule.
(iii) Hyperconjugation is of three types
(iv) Resonating structures due to hyperconjugation may be written involving “no bond” between the alpha carbon and hydrogen atoms.
\[H-\underset{H}{\mathop{\underset{|}{\mathop{\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,}}\,-CH=C{{H}_{2}}\underset{{}}{\longleftrightarrow}H-\underset{H}{\mathop{\underset{|}{\mathop{\overset{\overset{\oplus }{\mathop{H}}\,}{\mathop{\overset{{}}{\mathop{C}}\,}}\,}}\,}}\,=CH-\overset{}{\mathop{C}}\,{{H}_{2}}\underset{{}}{\longleftrightarrow}\]
\[\overset{\oplus}{\mathop{H}}\,\underset{H}{\mathop{\underset{|}{\mathop{\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,}}\,=CH-\overset{}{\mathop{C}}\,{{H}_{2}}\underset{{}}{\longleftrightarrow}H-\underset{\underset{\oplus}{\mathop{H}}\,}{\mathop{\underset{{}}{\mathop{\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,}}\,=CH-\overset{}{\mathop{C}}\,{{H}_{2}}\]
(v) Number of resonating structures due to the hyperconjugation = Number of \[\alpha \]-hydrogens + 1.
Applications of hyperconjugation
(1) Stability of alkenes : Hyperconjugation explains the stability of certain alkenes over other alkenes.
Stability of alkenes \[\propto \]Number of alpha hydrogens \[\propto \]Number of resonating structures
\[\xrightarrow[\text{Stability in decreasing order}]{C{{H}_{3}}-CH=C{{H}_{2}}>C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}>C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\mathop{CH-}}\,}}\,CH=C{{H}_{2}}}\]
(2) Carbon-carbon double bond length in alkenes : As we know that the more is the number of resonating structures, the more will be single bond character in carbon-carbon double bond.
(3) Stability of alkyl carbocations : Stability of alkyl carbocations µ number of resonating structures µ number of alpha hydrogens.
(4) Stability of alkyl free radicals : Stability of alkyl free radicals can be explained by hyperconjugation. Stability depends on the number of resonating structures.
(5) Electron releasing (or donating) power of R in alkyl benzene : \[C{{H}_{3}}-\](or alkyl group) is \[+R\] group, ortho-para directing group and activating group for electrophilic aromatic substitution reaction because of the hyperconjugation.
The electron donating power of alkyl group will depends on the number of resonating structures, this depends on the number of hydrogens present on \[\alpha -\]carbon. The electron releasing power of some groups are as follows,
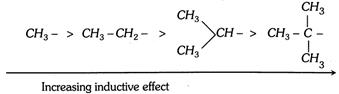
Electron donating power in decreasing order due to the hyperconjugation.
(6) Heat of hydrogenation : Hyperconjugation decreases the heat of hydrogenation.
(7) Dipole moment : Since hyperconjugation causes the development of charges, it also affects the dipole moment in the molecule.
The increase in dipole moment, when hydrogen of formaldehyde \[(\mu =2.27D)\] is replaced by methyl group, i.e., acetaldehyde \[(\mu =2.72D)\] can be referred to hyperconjugation, which leads to development of charges.
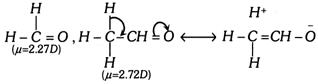
(8) Orienting influence of alkyl group in \[o,\,p\]-positions and of \[-CC{{l}_{3}}\] group in \[m\]-position : Ortho-para directing property of methyl group in toluene is partly due to \[+I\] effect and partly due to hyperconjugation.
Reverse Hyperconjugation : The phenomenon of hyperconjugation is also observed in the system given below,
\[-\underset{|}{\mathop{\overset{X}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,-C=C\]; where \[X=\] halogen
In such system the effect operates in the reverse direction. Hence the hyperconjugation in such system is known as reverse hyperconjugation.
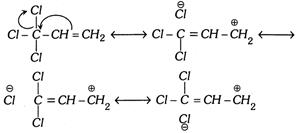
The meta directing influence and the deactivating effect of \[C{{X}_{3}}\] group in electrophilic aromatic substitution reaction can be explained by this effect.
You need to login to perform this action.
You will be redirected in
3 sec
