Hybridisation In Organic Compounds
Category : JEE Main & Advanced
(1) The process of mixing atomic orbitals to form a set of new equivalent orbitals is termed as hybridisation. There are three types of hybridisation,
(i) \[s{{p}^{3}}\] hybridisation (involved in saturated organic compounds containing only single covalent bonds),
(ii) \[s{{p}^{2}}\] hybridisation (involved in organic compounds having carbon atoms linked by double bonds) and
(iii) \[sp\] hybridisation (involved in organic compounds having carbon atoms linked by a triple bonds).
| Type of hybridisation | \[s{{p}^{3}}\] | \[s{{p}^{2}}\] | \[sp\] |
| Number of orbitals used | \[1s\] and \[3p\] | \[1s\] and \[2p\] | \[1s\] and \[1p\] |
| Number of unused p-orbitals | Nil | One | Two |
| Bond | Four \[-\sigma \] |
Three \[-\sigma \] One \[-\pi \] |
Two \[-\sigma \] Two\[-\pi \] |
| Bond angle | \[109.5{}^\circ \] | \[120{}^\circ \] | \[180{}^\circ \] |
| Geometry | Tetrahedral | Trigonal planar | Linear |
| % s-character | 25 or 1/4 | 33.33 or 1/3 | 50 or 1/2 |
(2) Determination of hybridisation at different carbon atoms : It can be done by two methods,
(i) First method : In this method hybridisation can be know by the number of \[\pi -\]bonds present on that particular atom.
| Number of \[\pi -\] bond/s | 0 | 1 | 2 |
| Type of hybridisation | \[s{{p}^{3}}\] | \[s{{p}^{2}}\] | \[sp\] |
Examples :
(i) \[\underset{_{s{{p}^{3}}}^{\downarrow }\,\,}{\mathop{C{{H}_{3}}}}\,-\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,\underset{_{s{{p}^{2}}}^{\ \downarrow }}{\overset{\ _{|\,|}^{O}}{\mathop{-C\,-}}}\,\underset{_{s{{p}^{3}}}^{\downarrow }\,\,}{\mathop{C{{H}_{3}}}}\,\]
(ii) \[\underset{_{s{{p}^{2}}}^{\downarrow \ }}{\mathop{C{{H}_{2}}}}\,=\underset{_{sp}^{\,\downarrow }}{\mathop{C}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{C{{H}_{2}}}}\,\]
(iii) \[\underset{_{s{{p}^{3}}}^{\downarrow }}{\mathop{\,\,\,C{{H}_{3}}}}\,-\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,-\underset{_{s{{p}^{3}}}^{\downarrow }\,\,}{\mathop{C{{H}_{2}}}}\,-\underset{\,_{sp}^{\downarrow }}{\mathop{C}}\,\equiv \underset{_{sp}^{\downarrow }}{\mathop{N}}\,\]
(iv) \[\underset{\,\,\,\,\,_{sp}^{\downarrow }}{\mathop{HC}}\,\equiv \underset{\,_{sp}^{\downarrow }}{\mathop{C}}\,-\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }\,\,}{\mathop{C{{H}_{2}}}}\,\]
(ii) Second method : (Electron pair method)
\[ep=bp+lp;\] where \[ep=\] electron pair present in hybrid orbitals , \[bp=\] bond pair present in hybrid orbitals
Number of \[bp=\] Number of atoms attached to the central atom of the species
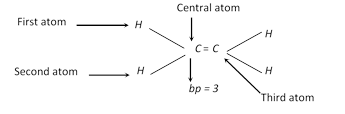
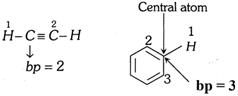
Number of lp’s can be determined as follows,
(a) If carbon has \[\pi \]- bonds or positive charge or odd electron, than lp on carbon will be zero.
(b) If carbon has negative charge, then lp will be equal to one.
Number of electron pairs (ep) tells us the type of hybridisation as follows,
| ep | 2 | 3 | 4 | 5 | 6 |
| Type of hybridisation | \[sp\] | \[s{{p}^{2}}\] | \[s{{p}^{3}}\] | \[s{{p}^{3}}d\] | \[s{{p}^{3}}{{d}^{2}}\] |
Example :
(i) \[\underset{\underset{\frac{\,lp=0}{ep=2,\,sp}}{\mathop{bp=2}}\,}{\mathop{C{{H}_{2}}=\underset{\downarrow \,\,\,\,}{\overset{\oplus \,\,\,\,\,}{\mathop{CH}}}\,}}\,\]
(ii) \[\underset{\underset{\frac{\,lp=1}{ep=3,\,s{{p}^{2}}}}{\mathop{bp=2}}\,}{\mathop{C{{H}_{2}}=\underset{\downarrow \,\,\,\,}{\overset{\Theta \,\,\,\,\,}{\mathop{CH}}}\,}}\,\]
(iii) \[\underset{\underset{\frac{\,lp=0}{ep=3,\,s{{p}^{2}}}}{\mathop{bp=3}}\,}{\mathop{C{{H}_{2}}=\underset{\begin{align}
& \,\,| \\
& \,C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\
\end{align}}{\overset{\,\bullet \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C-C{{H}_{3}}\,\,\,\,\,\,\,\,\,}}}\,}}\,\]
(iv)\[\underset{\begin{smallmatrix}
\,\,\,\,\,\,\,\,bp=1 \\
\frac{\,\,\,\,lp=1}{ep=2,\,sp}
\end{smallmatrix}}{\mathop{CH\equiv \overset{\Theta }{\mathop{\underset{\downarrow }{\mathop{C}}\,}}\,}}\,\]
(v) \[\underset{\begin{smallmatrix}
bp=3 \\
\frac{lp=1\,\,\,\,\,\,\,\,\,\,\,\,}{ep=4,\,s{{p}^{3}}}
\end{smallmatrix}}{\mathop{C{{H}_{3}}-\underset{\downarrow \,\,\,\,}{\mathop{\overset{\Theta \,\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}}}\,\]
(3) Applications of hybridisation
(i) Size of the hybrid orbitals : Since \[s\]- orbitals are closer to the nucleus than \[p\]- orbitals, it is reasonable to expect that greater the \[s\] character of an orbital the smaller it is. Thus the decreasing order of the size of the three hybrid orbitals is opposite to that of the decreasing order of \[s\] orbital character in the three hybrid orbitals.
\[s{{p}^{3}}>s{{p}^{2}}>sp\]
(ii) Electronegativity of different orbitals
(a) Electronegativity of s-orbital is maximum.
(b) Electronegativity of hybrid orbital \[\propto %\] s-character in hybrid orbitals
\[\underset{\,\,s\text{-character in decreasing order and electronegativity in decreasing order}}{\mathop{\xrightarrow{\ \ \ \ \ \underset{\text{ }\!\!%\!\!\text{ }s\text{ -character}}{\mathop{\text{Orbital}}}\,\text{ }\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{50}{\mathop{\,sp}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{33.33}{\mathop{\,\,s{{p}^{2}}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{25}{\mathop{\,\,\,s{{p}^{3}}}}\,\ \ \ \ \ \ \ \ \ \ \ \ }}}\,\]
Thus sp-hybrid carbon is always electronegative in character and \[s{{p}^{3}}\]- hybrid carbon is electropositive in character. \[s{{p}^{2}}\]-hybrid carbon can behave as electropositive (in carbocation) as well as electronegative (in carbanion) in character.
\[C{{H}_{3}}-\overset{\oplus \,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,\] \[C{{H}_{2}}=\overset{\oplus \,\,\,\,\,\,\,\,}{\mathop{CH}}\,\]
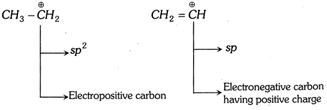
(c) Electronegativities of different hybrid and unhybrid orbitals in decreasing order is as follows
\[\underset{\text{ }\!\!%\!\!\text{ s-character and}\ \text{electronegativity in decreasing order}\text{.}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\ \,\,\,\,\,\,\,\,\,\,\ s>sp>s{{p}^{2}}>s{{p}^{3}}>p\ \ \ \,\ \ \ \ \,\,\,\,\,\,\,\,\,\,\,\,}}}\,\]
(iii) Bond length variation in hydrocarbons
% of s orbital character
\[\propto \frac{1}{C-C\,\text{bond length}}\propto \frac{1}{C-H\text{ bond length}}\]
| Bond type (C – H) | Bond length | Bond type (C – C) | Bond length |
| \[s{{p}^{3}}-s\](alkanes) | \[1.112{\AA}\] | \[s{{p}^{3}}-s{{p}^{3}}\] (alkanes) | \[1.54\text{ }{\AA}\] |
| \[s{{p}^{2}}-s\](alkenes) | \[1.103{\AA}\] | \[s{{p}^{2}}-s{{p}^{2}}\] (alkenes) | \[1.34{\AA}\] |
| \[sp-s\](alkynes) | \[1.08{\AA}\] | \[sp-sp\] (alkynes) | \[1.20{\AA}\] |
(iv) Bond strength in hydrocarbons : The shorter is the bond length, the greater is the compression between atomic nuclei and hence greater is the strength of that bond.
| Bond type (C – H) | Bond energy (kcal/mole) | Bond type (C – C) | Bond energy (kcal/mole) |
|
\[s{{p}^{3}}-s\] (in alkanes) |
104 |
\[s{{p}^{3}}-s{{p}^{3}}\] (in alkanes) |
80 – 90 |
|
\[s{{p}^{2}}-s\] (in alkenes) |
106 |
\[s{{p}^{2}}-s{{p}^{2}}\] (in alkenes) |
122 – 164 |
|
\[sp-s\] (in alkynes) |
121 |
\[sp-sp\] (in alkynes) |
123 – 199 |
(v) Acidity of hydrocarbons
(a) Hydrogen present on electronegative carbon is acidic in nature.
(b) Acidity of hydrogen is directly proportional to the electronegativity of the atom on which hydrogen is present.
Thus
\[\underset{\begin{smallmatrix}
\text{Electronegativity of the atoms } \\
\text{Acidity of compounds in decreasing order }
\end{smallmatrix}}{\mathop{\xrightarrow{H-O-H > N{{H}_{3}} > CH\equiv CH}}}\,\]
(c) Acidity of hydrocarbon µ % of s-character
\[\frac{\begin{matrix}
{} & CH\equiv CH & C{{H}_{2}}=C{{H}_{2}} & C{{H}_{3}}-C{{H}_{3}} \\
%\text{ s-character} & 50 & 33.33 & 25 \\
pKa & 25 & 44 & 50 \\
\end{matrix}}{s-\text{character and acidity in decreasing order}}\to \]
Acidity \[\propto Ka\] and Acidity \[\propto \frac{1}{pKa}(pKa=-\log Ka)\]
Order of acidic nature of alkynes is,
\[HC\equiv CH>HC\equiv C-C{{H}_{3}}\]
The relative acidic character follows the order;
\[{{H}_{2}}O>ROH>HC\equiv CH>N{{H}_{3}}>C{{H}_{2}}=C{{H}_{2}}>C{{H}_{3}}-C{{H}_{3}}\]
Obviously, the basic character of their conjugate bases follows the reverse order, i.e.,
\[C{{H}_{3}}CH_{2}^{}>C{{H}_{2}}=C{{H}^{}}>NH_{2}^{}>HC\equiv {{C}^{}}>R{{O}^{}}>H{{O}^{}}\]
You need to login to perform this action.
You will be redirected in
3 sec
