Electromeric Effect
Category : JEE Main & Advanced
(1) The phenomenon of movement of electrons from one atom to another in multibonded atoms at the demand of attacking reagent is called electromeric effect. It is denoted as E-effect and represented by a curved arrow (![]() ) showing the shifting of electron pair.
) showing the shifting of electron pair.
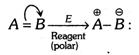
(2) (i)When the transfer of electrons take place towards the attacking reagent, the effect is called \[+E\] effect. The addition of acids to alkenes.
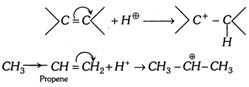
Since, \[-C{{H}_{3}}\] group is electron donating, the electrons are transferred in the direction shown.
The attacking reagent is attached to that atom on which electrons have been transferred.
(ii) When the transfer of electrons takes place away from the attacking reagent, the effect is called \[-E\] effect. Example, The addition of cyanide ion to carbonyl compounds.
![]()
The attacking reagent is not attached to that atom on which electrons have been transferred.
(3) Direction of the shift of electron pair : The direction of the shift of electron pair can be decided on the basis of following points.
(i) When the groups linked to a multiple bond are similar, the shift can occur in either direction.
(ii) When the dissimilar groups are linked on the two ends of the double bond, the shift is decided by the direction of inductive effect.
In the case of carbonyl group, the shift is always towards oxygen, i.e., more electronegative atom.
![]()
In cases where inductive effect and electromeric effect simultaneously operate, usually electrometric effect predominates.
You need to login to perform this action.
You will be redirected in
3 sec
