Conductor and Conductance
Category : NEET
Conductor and Conductance
Metallic and Electrolytic conductors.
(1) Conductors and Non – conductors: All substances do not conduct electrical current. The substances which allow the passage of electric current are called conductors. The best metal conductors are such as copper, silver, tin, etc. On the other hand, the substances which do not allow the passage of electric current through them are called non-conductors or insulators. Some common examples of insulators are rubber, wood, wax, etc.
(2) Types of conductors: The conductors are broadly classified into two types,
(i) Metallic conductors or electronic conductors.
(a) In metallic conductors, flow of electricity takes place without the decomposition of the substances.
(b) Flow of electricity is due to the flow of electrons only i.e., there is no flow of matter.
(c) In addition to metals, graphite and certain minerals also conduct electricity due to presence of free electrons in them, hence they are collectively called as electronic conductors.
(d) Metallic conduction decreases with increase of temperature. This is because kernels start vibrating which produce hinderance in the flow of electrons.
(e) The resistance offered by metals is also due to vibrating kernels.
(f) Metallic conductors obey Ohm's law.
(ii) Electrolytic conductors or Ionic conductors
(a) In electrolytic conductors flow of electricity takes place by the decomposition of the substance (Electrolyte).
(b) Flow of electricity is due to the movement of ions and hence there is flow of matter.
(c) Solutions of acids, bases and salts are the examples of electrolytic conductors.
(d) The electrolytic conduction will not occur unless the ions of the electrolyte are free to move. Therefore, these substances do not conduct electricity in the solid state but conduct electricity in the molten state or in their aqueous solutions.
(e) The electrical conduction increases with increase of temperature. This is generally due to increase in dissociation or decrease in the interionic attractions.
(f) The resistance shown by an electrolytic solution is due to factors like interionic attractions, viscosity of solvent etc.
(g) Electrolytic conductors also obey Ohm's law.
(h) All electrolytes do not ionise to the same extent in solution. On this basis, electrolytes are broadly divided into two types: strong electrolytes and weak electrolytes.
Strong electrolytes: The electrolytes which are almost completely dissociated into ions in solution are called strong electrolytes. For example, \[NaCl,KCl,HCl,NaOH,N{{H}_{4}}N{{O}_{3}},\]etc.
Weak electrolytes: The electrolytes which do not ionise completely in solution are called weak electrolytes. For example, \[C{{H}_{3}}COOH,{{H}_{2}}C{{O}_{3}},{{H}_{3}}B{{O}_{3}},HCN,HgC{{l}_{2}},ZnC{{l}_{2}},N{{H}_{4}}OH,\]etc. Thus in case of weak electrolytes, an equilibrium is established between the unionised electrolyte and the ions formed in solution. The extent of ionisation of a weak electrolyte is expressed in terms of degree of ionisation or degree of dissociation. It is defined as the fraction of total number of molecules of the electrolyte which ionise in the solution. It is generally denoted by alpha \[(\alpha ).\] For strong electrolytes, \[\alpha \] is almost equal to 1 and for weak electrolytes, it is always less than 1.
The electrical conductivity of the solutions of electrolytes depends upon the following factors,
(a) Interionic attractions: These depend upon the interactions between the ions of the solute molecules, i.e., solute-solute interactions. If the solute-solute interactions are large, the extent of dissociation will be less. These interactions are also responsible for the classification of electrolytes as strong electrolytes and weak electrolytes.
(b) Solvation of ions: These depend upon the interactions between the ions of the solute and the molecules of the solvent and are called solute-solvent interactions. If the solute-solvent interactions are strong, the ions of the solute will be highly solvated and their electrical conductivity will be low.
(c) Viscosity of the solvent: The viscosity of the solvent depends upon the solvent-solvent interactions. Larger the solvent-solvent interactions, larger will be the viscosity of the solvent and lower will be the electrical conductivity of the ions.
Factors affecting the electrolytic conductance.
In general, conductance of an electrolyte depends upon the following factors,
(1) Nature of electrolyte, (2) Concentration of the solution, (3) Temperature
(1) Nature of electrolyte: The conductance of an electrolyte depends upon the number of ions present in the solution. Therefore, the greater the number of ions in the solution the greater is the conductance. The number of ions produced by an electrolyte depends upon its nature. The strong electrolytes dissociate almost completely into ions in solutions and, therefore, their solutions have high conductance. On the other hand, weak electrolytes, dissociate to only small extents and give lesser number of ions. Therefore, the solutions of weak electrolytes have low conductance.
(2) Concentration of the solution: The molar conductance of electrolytic solution varies with the concentration of the electrolyte. In general, the molar conductance of an electrolyte increases with decrease in concentration or increase in dilution. The molar conductance of a few electrolytes in water at different concentrations are given in table
|
Z |
HCl |
KCl |
\[\mathbf{KN}{{\mathbf{O}}_{\mathbf{3}}}\] |
\[\mathbf{C}{{\mathbf{H}}_{\mathbf{3}}}\mathbf{COOH}\] |
\[\mathbf{N}{{\mathbf{H}}_{\mathbf{4}}}\mathbf{OH}\] |
|
0.1 |
391.3 |
129.0 |
120.4 |
5.2 |
3.6 |
|
0.05 |
399.1 |
133.4 |
126.3 |
– |
– |
|
0.01 |
412.0 |
141.3 |
132.8 |
16.3 |
11.3 |
|
0.005 |
415.8 |
143.5 |
131.5 |
– |
– |
|
0.001 |
421.4 |
146.9 |
141.8 |
49.2 |
34.0 |
|
0.0005 |
422.7 |
147.8 |
142.8 |
67.7 |
46.9 |
|
0(Infinite dilution) |
426.2 |
149.9 |
146.0 |
390.7 |
271.0 |
Inspection of table reveals that the molar conductance of strong electrolyte (\[HCl,KCl,KN{{O}_{3}})\] as well as weak electrolytes (\[C{{H}_{3}}COOH,N{{H}_{4}}OH)\]increase with decrease in concentration or increase in dilution. The variation is however different for strong and weak electrolytes.
Variation of conductivity with concentration for strong electrolytes: In case of strong electrolytes, there is a tendency for molar conductivity to approach a certain limiting value when the concentration approaches zero i.e., when the dilution is infinite. The molar conductivity when the concentration approaches zero (Infinite dilution) is called molar conductivity at infinite dilution. It is denoted by \[{{\Lambda }^{0}}.\]
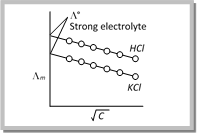
Thus, \[\Lambda ={{\Lambda }^{0}}\] when C \[\to 0\] (At infinite dilution)
It has been observed that the variation of molar conductivity with concentration may be given by the expression
\[\Lambda ={{\Lambda }^{0}}-A{{c}^{1/2}}\]
where, A is a constant and \[{{\Lambda }^{0}}\] is called molar conductivity at infinite dilution.
The variation of molar conductivity with concentration can be studied by plotting the values of \[{{\Lambda }_{m}}\] against square root of concentration \[(\sqrt{C})\]. The plots of variation of molar conductivity with \[\sqrt{C}\] for KCl and HCl are given in fig. It has been noticed that the variation of \[{{\Lambda }_{m}}\] with concentration, \[\sqrt{C}\] is small (Between 4 to 10% only) so that the plots can be extrapolated to zero concentration. This gives the limiting value of molar conductance when the concentration approaches zero, called molar conductivity at infinite dilution.
(ii) Variation of molar conductivity with concentration for weak electrolytes : The weak electrolytes dissociate to a much lesser extent as compared to strong electrolytes. Therefore, the molar conductivity is low as compared to that of strong electrolytes.
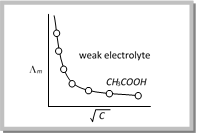
However, the variation of \[{{\Lambda }_{m}}\] with \[\sqrt{C}\] is very large and so much so that we cannot obtain molar conductance at infinite dilution \[({{\Lambda }^{0}})\] by extrapolation of the \[{{\Lambda }_{m}}\] versus \[\sqrt{C}\] plots. The behavior of weak electrolytes such as \[C{{H}_{3}}COOH\] is shown in figure.
Note: r The \[{{\Lambda }^{0}}\] value for weak electrolytes can be obtained by an indirect method based upon Kohlrausch law.
Explanation for the variation: The variation of molar conductance with concentration can be explained on the basis of conducting ability of ions for weak and strong electrolytes.
For weak electrolytes the variation of \[\Lambda \] with dilution can be explained on the bases of number of ions in solution. The number of ions furnished by an electrolyte in solution depends upon the degree of dissociation with dilution. With the increase in dilution, the degree of dissociation increases and as a result molar conductance increases. The limiting value of molar conductance \[({{\Lambda }^{0}})\] corresponds to degree of dissociation equal to 1 i.e., the whole of the electrolyte dissociates.
Thus, the degree of dissociation can be calculated at any concentration as,
\[\alpha =\frac{{{\Lambda }^{c}}}{{{\Lambda }^{0}}}\]
where \[\alpha \] is the degree of dissociation, \[{{\Lambda }^{c}}\] is the molar conductance at concentration C and \[{{\Lambda }^{0}}\] is the molar conductance at infinite dilution.
For strong electrolytes, there is no increase in the number of ions with dilution because strong electrolytes are completely ionised in solution at all concentrations (By definition). However, in concentrated solutions of strong electrolytes there are strong forces of attraction between the ions of opposite charges called inter-ionic forces. Due to these inter-ionic forces the conducting ability of the ions is less in concentrated solutions. With dilution, the ions become far apart from one another and inter-ionic forces decrease. As a result, molar conductivity increases with dilution. When the concentration of the solution becomes very-very low, the inter-ionic attractions become negligible and the molar conductance approaches the limiting value called molar conductance at infinite dilution. This value is characteristic of each electrolyte.
(3) Temperature: The conductivity of an electrolyte depends upon the temperature. With increase in temperature, the conductivity of an electrolyte increases.
You need to login to perform this action.
You will be redirected in
3 sec
