Collision theory, Energy of activation and Arrhenius equation
Category : NEET
Collision Theory, Energy of Activation and Arrhenius Equation
Theories of Reaction rate.
Some theories, which explain the reaction rate, are as follows:
(1) Collision theory
(i) The basic requirement for a reaction to occur is that the reacting species must collide with one another. This is the basis of collision theory for reactions.
(ii) The number of collisions that takes place per second per unit volume of the reaction mixture is known as collision frequency (Z). The value of collision frequency is very high of the order of \[{{10}^{25}}\,\text{to }{{10}^{28}}\] in case of binary collisions.
(iii) Every collision does not bring a chemical change. The collisions that actually produce the product are effective collisions. The effective collisions, which bring chemical change, are few in comparison to the total number of collisions. The collisions that do not form a product are ineffective elastic collisions, i.e., molecules just collide and disperse in different directions with different velocities.
(iv) For a collision to be effective, the following two barriers are to be cleared.
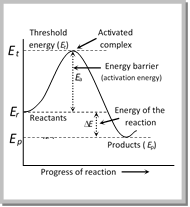
(a) Energy barrier : “The minimum amount of energy which the colliding molecules must possess as to make the chemical reaction to occur, is known as threshold energy”.
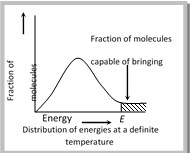
(b) Orientation barrier : The colliding molecules should also have proper orientation so that the old bonds may break and new bonds are formed. For example, \[N{{O}_{2}}(g)+N{{O}_{2}}(g)\to {{N}_{2}}{{O}_{4}}(g).\] During this reaction, the products are formed only when the colliding molecules have proper orientation at the time of collisions. These are called effective collisions.
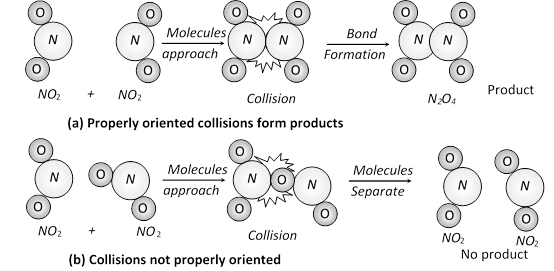
(v) Thus, the main points of collision theory are as follows,
(a) For a reaction to occur, there must be collisions between the reacting species.
(b) Only a certain fraction of the total number of collisions is effective in forming the products.
(c) For effective collisions, the molecules should possess sufficient energy as well as orientation.
(vi) The fraction of effective collisions, under ordinary conditions may vary from nearly zero to about one for ordinary reactions. Thus, the rate of reaction is proportional to :
(a) The number of collisions per unit volume per second (Collision frequency, Z) between the reacting species
(b) The fraction of effective collisions (Properly oriented and possessing sufficient energy), f
i.e., \[\text{Rate}=\frac{-dx}{dt}=f\times Z\]; Where f is fraction of effective collision and Z is the collision frequency.
(vii) The physical meaning of the activation energy is that it is the minimum relative kinetic energy which the reactant molecules must possess for changing into the products molecules during their collision. This means that the fraction of successful collision is equal to \[{{e}^{-{{E}_{a}}/RT}}\] called Boltzmann factor.
(viii) It may be noted that besides the requirement of sufficient energy, the molecules must be properly oriented in space also for a collision to be successful. Thus, if \[{{Z}_{AB}}\] is the collision frequency, P is the orientation factor (Steric factor) then, \[k=P{{Z}_{AB}}.{{e}^{-{{E}_{a}}/RT}}\]. If we compare this equation with Arrhenius equation. \[k=A\,{{e}^{-{{E}_{a}}/RT}}\]
We know that pre-exponential form 'A' in Arrhenius equation is, \[A=P{{Z}_{AB}}\].
Concept of activation energy
(i) The excess energy (Over and above the average energy of the reactants) which must be supplied to the reactants to undergo chemical reactions is called activation energy\[({{E}_{a}})\],\[{{E}_{a}}={{E}_{\text{(Threshold}\,\text{energy)}}}-\,\ {{E}_{\text{(Re}\text{ac}\text{tan}\text{ts)}}}\]
Activation energy = Threshold energy – Average kinetic energy of the reacting molecules.
(a) Zero activation energy = Fraction of effective collision (f) will be very large = Very fast reaction (Instantaneous reaction).
(b) Low activation energies = Fraction of effective collision (f) will be large = Fast reactions.
(c) High activation energies = Fraction of effective collision (f) will be small = Slow reaction.
(ii) When the colliding molecules possess the kinetic energy equal to activation energy, the atomic configuration of species formed at this stage is different from the reactants as well as products. This stage is called the activated state or transition state and specific configuration of this state is called activated complex. In other words, we can say that, A collision between high energy molecules overcomes the forces of repulsion and brings the formation of an unstable molecule cluster called the activated complex. The life span of an activated complex is very small. Thus, the activated complex breaks either into reactants again or new substances, i.e., products.
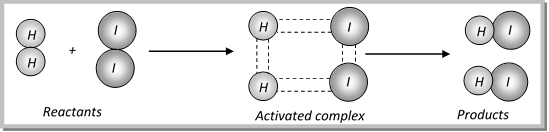
(iii) The activation energy \[({{E}_{a}})\] depends upon the nature of chemical bonds undergoing rupture and is independent of enthalpies of reactants and products.
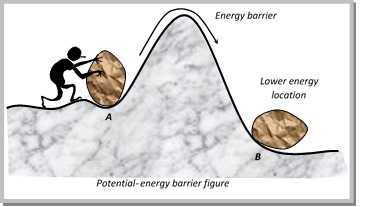
(iv) According to the concept of activation energy, the reactants do not change directly into the products. The reactant first absorb energy equal to activation energy and form activated complex. At this state, the molecules must have energy at least equal to the threshold energy. This means that the reaction involves some energy barrier which must be overcome before products are formed. The energy barrier is known as activation energy barrier.
![]()
Note : q The activation energy is found to increase with the lowering of temperature i.e., at lower temperatures the activation energy tends to increase.
(i) According to transition state theory the activated complex is supposed to be in equilibrium with the reactant molecules.
(ii) Once the transition state is formed it can either return to the initial reactants or proceeds to form the products.
(iii) Assuming that once formed the transition state proceeds to products we can say that rate is proportional to concentration of transition state. Mathematically, Rate \[\propto \]Transition state; Rate= Constant × Transition state
(iv) The activation energy for the forward reaction, \[(E_{a}^{f})\]and the activation energy for the reverse reaction \[(E_{a}^{r})\] are related to the enthalpy \[(\Delta H)\]of the reaction by the equation \[\Delta H=E_{a}^{f}-E_{a}^{r}\].
(a) For endothermic reactions, \[\Delta H>0,\] so that \[E_{a}^{r}<E_{a}^{f}\]
(b) For exothermic reaction, \[\Delta H<0,\] so that \[E_{a}^{r}>E_{a}^{f}\].
.![]()
Note : q Exothermic reaction requires less activation energy than the endothermic reaction.Therefore an exothermic reaction
proceeds at a faster rate than the endothermic reaction.
q Kinetic stability of fuels : Combustion of fuels is highly exothermic reaction yet these can be safely stored in contact with oxygen or air. The stability of fuels is due to high activation energy of these combustion reactions.
q Ea cannot be zero (if suppose Ea =0 then according to Arrhenius equation k = A i.e., every collision between molecules leads to be chemical reaction. This is not true.)
Arrhenius equation and Calculation of activation energy.
Arrhenius proposed a quantitative relationship between rate constant and temperature as,
\[k=A\,{{e}^{-{{E}_{a}}/RT}}\] …..(i)
The equation is called Arrhenius equation in which constant A is known as frequency factor. This factor is related to number of binary molecular collision per second per litre. \[{{E}_{a}}\] is the activation energy. T is the absolute temperature and R is the gas constant. Both A and \[{{E}_{a}}\] are collectively known as Arrhenius parameters. Taking logarithm equation (i) may be written as,
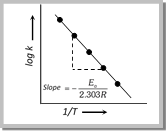
\[\log k=\log A-\frac{{{E}_{a}}}{2.303\,RT}\] …..(ii)
The value of activation energy \[({{E}_{a}})\] increases, the value of k decreases and therefore, the reaction rate decreases. When log k plotted against \[\frac{1}{T},\] we get a straight line. The intercept of this line is equal to log A and slope equal to \[\frac{-{{E}_{a}}}{2.303\,R}\]. Therefore \[{{E}_{a}}=-2.303\,R\times \text{slope}\].
Rate constants for the reaction at two different temperatures \[{{T}_{1}}\] and \[{{T}_{2}}\],
\[\log \frac{{{k}_{2}}}{{{k}_{1}}}=\frac{{{E}_{a}}}{2.303R}\left[ \frac{1}{{{T}_{1}}}-\frac{1}{{{T}_{2}}} \right]\] …..(iii)
where \[{{k}_{1}}\]and \[{{k}_{2}}\]are rate constant at temperatures \[{{T}_{1}}\] and \[{{T}_{2}}\] respectively \[({{T}_{2}}>{{T}_{1}})\]..
![]()
Note : q Generally rate of reaction increases with increase in temperature but remember for the reaction
2NO + O2 ® 2NO2; the rate decreases slightly with increase in temperature because it has small negative temperature coefficient.
q When Ea= 0, the rate of reaction becomes independent of temperature (Ea= activation energy)
Examples based on Arrhenius equation and Calculation of activation energy
Example : 17 In the Arrhenius equation for a certain reaction, the values of A and \[{{E}_{a}}\] are \[4\times {{10}^{13}}{{\sec }^{-1}}\] and \[9.86\,kJ\,mo{{l}^{-1}}\] respectively. If the reaction is of first order, at what temperature will its half-life period be 10 minute
[IIT 1990]
Solution : (a) According to Arrhenius equation, \[k=A{{e}^{-{{E}_{a}}/RT}}\] or \[\log \frac{k}{A}=-\frac{{{E}_{a}}}{RT}\times \frac{1}{2.303}\]
\[k=\frac{0.693}{{{t}_{1/2}}}=\frac{0.693}{10\times 60}=1.155\times {{10}^{-3}}\]
\[\therefore \,\,\log \frac{1.155\times {{10}^{-3}}}{4\times {{10}^{13}}}=-\frac{98.6\times {{10}^{3}}}{8.314\times T\times 2.303\,}\] or \[-16.54=-\frac{98600}{8.314\times 2.303\,T}\]
or \[T=\frac{98600}{8.314\times 2.303\times 16.54}=311.34K.\]
Example : 18 A first order reaction is 50% completed in 30 minutes at 27°C and in 10 minutes at 47°C. Calculate the activation energy of the reaction. [IIT 1988]
(a) \[46.8\,kJ\,mo{{l}^{-1}}\] (b) \[43.8\,kJ\,mo{{l}^{-1}}\] (c) \[50.8\,kJ\,mo{{l}^{-1}}\] (d) \[60.8\,KJ\,mo{{l}^{-1}}\]
Solution : (b) Let us first calculate \[{{k}_{1}}\] and \[{{k}_{2}}\] at temperatures 27°C and 47°C. We know that \[{{t}_{1/2}}=\frac{0.693}{k}\] or \[k=\frac{0.693}{{{t}_{1/2}}}\]
At 27°C, \[{{t}_{1/2}}=30\min \]; \[{{k}_{1}}=\frac{0.693}{30}=0.0231\]
At 47°C, \[{{t}_{1/2}}=10\min \]; \[{{k}_{2}}=\frac{0.693}{10}=0.0693\]
Now, \[\log \frac{{{k}_{2}}}{{{k}_{1}}}=\frac{{{E}_{a}}}{2.303R}\left[ \frac{1}{{{T}_{1}}}-\frac{1}{{{T}_{2}}} \right]\]; \[\log \frac{0.0693}{0.0231}=\frac{{{E}_{a}}}{2.303\times 8.314}\left[ \frac{1}{300}-\frac{1}{320} \right]\]
\[\log 3=\frac{{{E}_{a}}}{2.303\times 8.314}\left[ \frac{20}{300\times 320} \right]\] \[\Rightarrow \,\,0.4771=\frac{{{E}_{a}}\times 20}{2.303\times 8.314\times 300\times 320}\]
or \[{{E}_{a}}=\frac{0.4771\times 2.303\times 8.314\times 300\times 320}{20}\] \[=43848Jmo{{l}^{-1}}\,\text{or}\,43.8\,kJ\,mo{{l}^{-1}}\].
Example: 19 The rate of reaction becomes 2 times for every \[{{10}^{o}}C\] rise in temperature. How the rate of reaction will increases when temperature is increased from \[{{30}^{o}}C\] to \[{{80}^{o}}C\]
(a) 16 (b) 32 (c) 64 (d) 128
Solution: (b) \[\frac{{{k}_{t}}+10}{{{k}_{t}}}=\frac{{{r}_{t}}+10}{{{r}_{t}}}=2\].
For an increase of temperature to \[{{50}^{o}}C\], i.e., 5 times, the rate increases by \[{{2}^{5}}\] times, i.e., 32 times.
Example: 20 The rate constant, the activation energy and the Arrhenius parameter of a chemical reaction at \[{{25}^{o}}C\] are \[3.0\times {{10}^{-4}}{{s}^{-1}},\] 104.4 \[kJ\,mo{{l}^{-1}}\] and \[6.0\times {{10}^{14}}{{s}^{-1}}\] respectively. The value of the rate constant as \[T\to \infty \] is
[IIT 1996]
(a) \[2.0\times {{10}^{18}}{{s}^{-1}}\] (b) \[6.0\times {{10}^{14}}{{s}^{-1}}\] (c) Infinity (d) \[3.6\times {{10}^{30}}{{s}^{-1}}\]
Solution: (b) \[k=A{{e}^{-Ea/RT}}\]; At \[T\to \infty \] i.e., \[\frac{1}{T}\to 0\]; \[k=A=6\times {{10}^{14}}{{\sec }^{-1}}\]
Example: 21 The activation energy of a reaction is \[9kcal/mole\]. The increase in the rate constant when its temperature is raised from 295 to 300 K is approximately [Pb. CET 1988]
(a) 10% (b) 50% (c) 100% (d) 28.8%
Solution: (d) \[\log \frac{{{k}_{2}}}{{{k}_{1}}}=\frac{{{E}_{a}}}{2.303\,R}\,\left[ \frac{{{T}_{2}}-{{T}_{1}}}{{{T}_{1}}{{T}_{2}}} \right]\]= \[\frac{9000}{2.303\times 2}\,\left[ \frac{300-295}{295\times 300} \right]\]= \[0.1103\]
Hence \[\frac{{{k}_{2}}}{{{k}_{1}}}=1.288\] or \[{{k}_{2}}=1.288\,{{k}_{1}}\] i.e., increase = 28.8%.
.
You need to login to perform this action.
You will be redirected in
3 sec
