Some Commercially Important Aliphatic Carbonyl Compounds
Category : JEE Main & Advanced
Formaldehyde : Formaldehyde is the first member of the aldehyde series. It is present in green leaves of plants where its presence is supposed to be due to the reaction of \[C{{O}_{2}}\] with water in presence of sunlight and chlorophyll.
(1) Preparation
(i) \[2C{{H}_{3}}OH+{{O}_{2}}\underset{300-400{}^\circ C}{\mathop{\xrightarrow{\text{Platinised asbestos}}}}\,\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
\[C{{H}_{3}}OH+[O]\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,\ HCHO+{{H}_{2}}O\]
(ii) \[C{{H}_{3}}OH\underset{300-400{}^\circ C}{\mathop{\xrightarrow{Cu\ \text{ or }Ag}}}\,\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
(iii) \[\underset{\text{Calcium}\ \text{formate}}{\mathop{Ca{{(HCOO)}_{2}}}}\,\xrightarrow{\text{Heat}}\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
(iv) \[C{{H}_{2}}=C{{H}_{2}}+{{O}_{3}}\]\[\underset{Pd}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
(v) \[\underset{\text{Methane}}{\mathop{C{{H}_{4}}}}\,+{{O}_{2}}\underset{Catalyst}{\mathop{\xrightarrow{\text{Mo-oxide}}}}\,\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
(vi) \[CO+{{H}_{2}}\xrightarrow{\text{Elec}\text{. discharge}}\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
(2) Physical properties
(i) It is a colourless, pungent smelling gas.
(ii) It is extremely soluble in water. Its solubility in water may be due to hydrogen bonding between water molecules and its hydrate.
(iii) It can easily be condensed into liquid. The liquid formaldehyde boils at \[-{{21}^{o}}C\].
(iv) It causes irritation to skin, eyes, nose and throat.
(v) Its solution acts as antiseptic and disinfectant.
(3) Uses
(i) The 40% solution of formaldehyde (formalin) is used as disinfectant, germicide and antiseptic. It is used for the preservation of biological specimens.
(ii) It is used in the preparation of hexamethylene tetramine (urotropine) which is used as an antiseptic and germicide.
(iii) It is used in silvering of mirror.
(iv) It is employed in manufacture of synthetic dyes such as para-rosaniline, indigo, etc.
(v) It is used in the manufacture of formamint (by mixing formaldehyde with lactose) – a throat lozenges.
(vi) It is used for making synthetic plastics like bakelite, urea-formaldehyde resin, etc.
(vii) Rongalite – a product obtained by reducing formaldehyde sodium bisulphite derivative with zinc dust and ammonia and is used as a reducing agent in vat dyeing.
Acetaldehyde
Acetaldehyde is the second member of the aldehyde series. It occurs in certain fruits. It was first prepared by Scheele in 1774 by oxidation of ethyl alcohol.
(1) Preparation : It may be prepared by any of the general methods. The summary of the methods is given below
(i) By oxidation of ethyl alcohol with acidified potassium dichromate or with air in presence of a catalyst like silver at \[{{300}^{o}}C\].
(ii) By dehydrogenation of ethyl alcohol. The vapours of ethyl alcohol are passed over copper at \[{{300}^{o}}C\].
(iii) By heating the mixture of calcium acetate and calcium formate.
(iv) By heating ethylidene chloride with caustic soda or caustic potash solution.
(v) By the reduction of acetyl chloride with hydrogen in presence of a catalyst palladium suspended in barium sulphate (Rosenmund's reaction).
(vi) By the reduction of \[C{{H}_{3}}CN\] with stannous chloride and HCl in ether and hydrolysis (Stephen's method).
(vii) By hydration of acetylene with dil. \[{{H}_{2}}S{{O}_{4}}\] and \[HgS{{O}_{4}}\] at \[{{60}^{o}}C\].
(viii) By ozonolysis of butene-2 and subsequent breaking of ozonide.
(ix) Laboratory preparation : Acetaldehyde is prepared in the laboratory by oxidation of ethyl alcohol with acidified potassium dichromate or acidified sodium dichromate.
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}+4{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}{{K}_{2}}S{{O}_{4}}+C{{r}_{2}}{{(S{{O}_{4}})}_{3}}+4{{H}_{2}}O+3[O]\]
\[[C{{H}_{3}}C{{H}_{2}}OH+O\xrightarrow{{}}C{{H}_{3}}CHO+{{H}_{2}}O]\times 3\]
\[\underset{\begin{smallmatrix}\text{Potassium} \\\text{dichromate}\end{smallmatrix}}{\mathop{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}\,+\underset{\text{Ethyl}\,\text{alcohol}}{\mathop{3C{{H}_{3}}C{{H}_{2}}OH}}\,+\underset{\text{Sulphuric acid}}{\mathop{4{{H}_{2}}S{{O}_{4}}}}\,\xrightarrow{{}}\] \[\underset{\begin{smallmatrix}\text{Potassium}\\\text{sulphate}\end{smallmatrix}}{\mathop{{{K}_{2}}S{{O}_{4}}}}\,+\underset{\begin{smallmatrix}\text{Chromic}\\\text{sulphate}\end{smallmatrix}}{\mathop{C{{r}_{2}}{{(S{{O}_{4}})}_{3}}}}\,+\underset{\text{Acetaldehyde}}{\mathop{3C{{H}_{3}}CHO}}\,+\underset{\text{Water}}{\mathop{7{{H}_{2}}O}}\,\]
To recover acetaldehyde, the distillate is treated with dry ammonia when crystallised product, acetaldehyde ammonia, is formed. It is filtered and washed with dry ether. The dried crystals are then distilled with dilute sulphuric acid when pure acetaldehyde is collected.
\[C{{H}_{3}}CHO+N{{H}_{3}}\to \,\underset{\text{Acetaldehyde ammonia}}{\mathop{\overset{OH}{\mathop{C{{H}_{3}}\overset{|\ \ \ \ }{\mathop{-CH-}}\,N{{H}_{2}}}}\,}}\,\xrightarrow{{{H}_{2}}S{{O}_{4}}}\] \[\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\ +{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\]
(x) Manufacture : Acetaldehyde can be manufactured by one of the following methods:
(a) By air oxidation of ethyl alcohol
\[2C{{H}_{3}}C{{H}_{2}}OH+{{O}_{2}}\underset{300{}^\circ C}{\mathop{\xrightarrow{Ag}}}\,2C{{H}_{3}}CHO+2{{H}_{2}}O\]
(b) By dehydrogenation of alcohol
\[C{{H}_{3}}C{{H}_{2}}OH\underset{300{}^\circ C}{\mathop{\xrightarrow{Cu}}}\,C{{H}_{3}}CHO\]
(c) By hydration of acetylene
\[CH\equiv CH+{{H}_{2}}O\underset{{{H}_{2}}S{{O}_{4}}(40%)}{\mathop{\xrightarrow{HgS{{O}_{4}},(1%),60{}^\circ C}}}\,C{{H}_{3}}CHO\]
(d) From ethylene (Wacker process)
\[{{H}_{2}}C=C{{H}_{2}}+{{O}_{2}}\underset{{{H}_{2}}O}{\mathop{\xrightarrow{PdC{{l}_{2}},CuC{{l}_{2}}}}}\,{{H}_{3}}C-CHO\]
(2) Physical properties
(i) Acetaldehyde is a colourless volatile liquid. It boils at \[{{21}^{o}}C\].
(ii) It has a characteristic pungent smell.
(iii) It is soluble in water, chloroform, ethyl alcohol and ether. Its aqueous solution has a pleasant odour. In water, it is hydrated to a considerable extent to form ethylidene diol.
\[C{{H}_{3}}CHO+{{H}_{2}}O\xrightarrow{{}}C{{H}_{3}}CH{{(OH)}_{2}}\]
(3) Uses : Acetaldehyde is used :
(i) In the preparation of acetic acid, acetic anhydride, ethyl acetate, chloral, 1,3-butadiene (used in rubbers), dyes and drugs.
(ii) As an antiseptic inhalent in nose troubles.
(iii) In the preparation of paraldehyde (hypnotic and sporofic) and metaldehyde (solid fuel).
(iv) In the preparation of acetaldehyde ammonia (a rubber accelerator).
Comparative Study Of Formaldehyde And Acetaldehyde
| S.No. | Reaction | Formaldehyde HCHO | Acetaldehyde \[\mathbf{C}{{\mathbf{H}}_{\mathbf{3}}}\mathbf{CHO}\] | ||
| Similarities | |||||
| 1. |
Addition of hydrogen (a) \[{{H}_{2}}\] in presence of catalyst, Ni, Pd or Pt (b) \[LiAl{{H}_{4}}\] (ether) (c) Amalgamated zinc + conc. HCl (Clemmenson reduction) |
Forms methyl alcohol \[HCHO+{{H}_{2}}\xrightarrow{{}}C{{H}_{3}}OH\] Forms methyl alcohol Forms methane \[HCHO+4H\xrightarrow{{}}C{{H}_{4}}+{{H}_{2}}O\] |
Forms ethyl alcohol \[C{{H}_{3}}CHO+{{H}_{2}}\xrightarrow{{}}C{{H}_{3}}C{{H}_{2}}OH\] Forms ethyl alcohol Forms ethane \[C{{H}_{3}}CHO+4H\xrightarrow{{}}{{C}_{2}}{{H}_{6}}+{{H}_{2}}O\] |
||
| 2. | Addition of \[NaHS{{O}_{3}}\] solution |
Forms bisulphite addition product \[HCHO+NaHS{{O}_{3}}\xrightarrow{{}}C{{H}_{2}}(OH)S{{O}_{3}}Na\] |
Forms bisulphite addition product \[C{{H}_{3}}CHO+NaHS{{O}_{3}}\xrightarrow{{}}\] \[C{{H}_{3}}CH(OH)S{{O}_{3}}Na\] |
||
| 3. | Addition of HCN | Forms formaldehyde cyanohydrin \[HCHO+HCN\xrightarrow{{}}C{{H}_{2}}(OH)CN\] | Forms acetaldehyde cyanohydrin \[C{{H}_{3}}CHO+HCN\xrightarrow{{}}\] \[C{{H}_{3}}CH(OH)CN\] | ||
| 4. | Addition of Grignard reagent followed by hydrolysis |
Forms ethyl alcohol
\[\underset{-Mg(OH)I}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,C{{H}_{3}}C{{H}_{2}}OH\] |
Forms isopropyl alcohol \[C{{H}_{3}}CHO+C{{H}_{3}}MgI\xrightarrow{{}}\] \[\underset{\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}\underset{\,\,\,|}{\mathop{-C}}\,HOMgI}}\,\underset{-Mg(OH)I}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\] \[\underset{\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}\underset{|\ \ \ \ }{\mathop{-CH-}}\,OH}}\,\] |
||
| 5. |
With hydroxylamine \[N{{H}_{2}}OH\] |
Forms formaldoxime \[C{{H}_{2}}=O+{{H}_{2}}NOH\xrightarrow{-{{H}_{2}}O}\] \[C{{H}_{2}}=NOH\] |
Forms acetaldoxime \[C{{H}_{3}}CH=O+{{H}_{2}}NOH\xrightarrow{-{{H}_{2}}O}\] \[C{{H}_{3}}CH=NOH\] |
||
| 6. |
With hydrazine \[(N{{H}_{2}}N{{H}_{2}})\] |
Forms formaldehyde hydrazone \[C{{H}_{2}}O+{{H}_{2}}N\ N{{H}_{2}}\xrightarrow{-{{H}_{2}}O}\] \[C{{H}_{2}}=NN{{H}_{2}}\] |
Forms acetaldehyde hydrazone \[C{{H}_{3}}CH=O+{{H}_{2}}NN{{H}_{2}}\xrightarrow{-{{H}_{2}}O}\] \[C{{H}_{3}}CH=NN{{H}_{2}}\] |
||
| 7. |
With phenyl hydrazine \[({{C}_{6}}{{H}_{5}}NHN{{H}_{2}})\] |
Forms formaldehyde phenyl hydrazone \[C{{H}_{2}}=O+{{H}_{2}}NNH{{C}_{6}}{{H}_{5}}\xrightarrow{-{{H}_{2}}O}\] \[C{{H}_{2}}=NNH{{C}_{6}}{{H}_{5}}\] |
Forms acetaldehyde phenyl hydrazone \[C{{H}_{3}}CH=O+{{H}_{2}}NNH{{C}_{6}}{{H}_{5}}\] \[\xrightarrow{-{{H}_{2}}O}C{{H}_{3}}CH=NNH{{C}_{6}}{{H}_{5}}\] |
||
| 8. |
With semicarbazide \[({{H}_{2}}NNHCON{{H}_{2}})\] |
Forms formaldehyde semicarbazone \[C{{H}_{2}}=O+{{H}_{2}}NNHCON{{H}_{2}}\xrightarrow{-{{H}_{2}}O}\] \[C{{H}_{2}}=NNHCON{{H}_{2}}\] |
Forms acetaldehyde semicarbazone \[C{{H}_{3}}CH=O+{{H}_{2}}NNHCON{{H}_{2}}\] \[\xrightarrow{-{{H}_{2}}O}C{{H}_{3}}CH=NNHCON{{H}_{2}}\] |
||
| 9. |
With alcohol \[({{C}_{2}}{{H}_{5}}OH)\] in presence of acid |
Forms ethylal \[{{H}_{2}}C=O+2{{C}_{2}}{{H}_{5}}OH\xrightarrow{HCl}\] |
Forms acetaldehyde diethyl acetal \[C{{H}_{3}}CHO+2{{C}_{2}}{{H}_{5}}OH\xrightarrow{HCl}\] |
||
| 10. |
With thioalcohols \[({{C}_{2}}{{H}_{5}}SH)\]in presence of acid |
Forms thio ethylal \[{{H}_{2}}C=O+2{{C}_{2}}{{H}_{5}}SH\xrightarrow{{}}\] |
Forms acetaldehyde diethyl thioacetal \[C{{H}_{3}}CH=O+2{{C}_{2}}{{H}_{5}}SH\xrightarrow{{}}\] |
||
| 11. |
Oxidation with acidified \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] |
Forms formic acid \[HCHO+O\xrightarrow{{}}HCOOH\] |
Forms acetic acid \[C{{H}_{3}}CHO+O\xrightarrow{{}}C{{H}_{3}}COOH\] |
||
| 12. | With Schiff's reagent | Restores pink colour of Schiff's reagent | Restores pink colour of Schiff's reagent | ||
| 13. | With Tollen's reagent |
Gives black precipitate of Ag or silver mirror \[A{{g}_{2}}O+HCHO\xrightarrow{{}}2Ag+HCOOH\] |
Gives black precipitate of Ag or silver mirror \[A{{g}_{2}}O+C{{H}_{3}}CHO\xrightarrow{{}}\] \[2Ag+C{{H}_{3}}COOH\] |
||
| 14. | With Fehling's solution or Benedict's solution |
Gives red precipitate of cuprous oxide \[2CuO+HCHO\xrightarrow{{}}C{{u}_{2}}O+HCOOH\] |
Gives red precipitate of cuprous oxide \[2CuO+C{{H}_{3}}CHO\xrightarrow{{}}\] \[C{{u}_{2}}O+C{{H}_{3}}COOH\] |
||
| 15. | Polymerisation |
Undergoes polymerisation
|
Undergoes polymerisation
|
||
| Dissimilarities | |||||
| 16. |
With \[PC{{l}_{5}}\] |
No reaction |
Forms ethylidene chloride
\[+POC{{l}_{3}}\] |
||
| 17. | With chlorine | No reaction |
Forms chloral \[C{{H}_{3}}CHO+3C{{l}_{2}}\xrightarrow{{}}CC{{l}_{3}}CHO\] \[+3HCl\] |
||
| 18. |
With \[Se{{O}_{2}}\] |
No reaction |
Forms glyoxal \[C{{H}_{3}}CHO+Se{{O}_{2}}\xrightarrow{{}}CHO.CHO\] \[+Se+{{H}_{2}}O\] |
||
| 19. |
Iodoform reaction \[({{I}_{2}}+NaOH)\] |
No reaction |
Forms iodoform \[C{{H}_{3}}CHO+3{{I}_{2}}+4NaOH\xrightarrow{{}}\] \[CH{{l}_{3}}+HCOONa+3NaI+3{{H}_{2}}O\] |
||
| 20. | With dil. alkali (Aldol condensation) | No reaction |
Forms aldol \[C{{H}_{3}}CHO+HC{{H}_{2}}CHO\xrightarrow{{}}\] \[C{{H}_{3}}CH(OH)C{{H}_{2}}CHO\] |
||
| 21. | With conc. NaOH (Cannizzaro's reaction) |
Forms sodium formate and methyl alcohol \[2HCHO+NaOH\xrightarrow{{}}HCOONa\]\[+C{{H}_{3}}OH\] |
Forms a brown resinous mass | ||
| 22. | With ammonia |
Forms hexamethylene tetramine (urotropine) \[6HCHO+4N{{H}_{3}}\xrightarrow{{}}{{(C{{H}_{2}})}_{6}}{{N}_{4}}+6{{H}_{2}}O\] |
Forms addition product, acetaldehyde ammonia \[C{{H}_{3}}CHO+N{{H}_{3}}\xrightarrow{{}}\]
|
||
| 23. | With phenol | Forms bakelite plastic | No reaction | ||
| 24. | With urea | Forms urea-formaldehyde plastic | No reaction | ||
| 25. |
Condensation in presence of \[Ca{{(OH)}_{2}}\] |
Form formose (a mixuture of sugars) | No reaction | ||
Inter conversion of formaldehyde and acetaldehyde
(1) Ascent of series : Conversion of formaldehyde into acetaldehyde
(i) \[\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\xrightarrow{{{H}_{2}}/Ni}\underset{\begin{smallmatrix} \text{Methyl} \\ \text{alcohol} \end{smallmatrix}}{\mathop{C{{H}_{3}}OH}}\,\xrightarrow{PC{{l}_{5}}}\underset{\begin{smallmatrix} \text{Methyl} \\ \text{chloride} \end{smallmatrix}}{\mathop{C{{H}_{3}}Cl}}\,\underset{KCN}{\mathop{\xrightarrow{\text{Alc}\text{.}}}}\,\]
\[\underset{\begin{smallmatrix} \text{Methyl} \\ \text{cyanide} \end{smallmatrix}}{\mathop{C{{H}_{3}}CN}}\,\xrightarrow{Na/\text{Alcohol}}\underset{\text{Ethyl amine}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,\underset{HCl}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\]
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}\text{(dil}\text{.)}}}}\,\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\]
(ii) \[\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\underset{\text{Ether}}{\mathop{\xrightarrow{C{{H}_{3}}MgI}}}\,C{{H}_{3}}C{{H}_{2}}OMgI\xrightarrow{{{H}_{3}}{{O}^{+}}}\]
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{300{}^\circ C}{\mathop{\xrightarrow{Cu}}}\,\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\]
(iii) \[\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,\underset{\text{Formic acid}}{\mathop{HCOOH}}\,\xrightarrow{Ca{{(OH)}_{2}}}\]
\[\underset{\text{Calcium formate}}{\mathop{{{(HCOO)}_{2}}Ca}}\,\underset{\text{heat}}{\mathop{\xrightarrow{{{(C{{H}_{3}}COO)}_{2}}Ca}}}\,\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\]
(2) Descent of series : Conversion of acetaldehyde into formaldehyde
(i) \[\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\xrightarrow{N{{H}_{3}}}\]
\[\underset{\text{Amm}\text{. acetate}}{\mathop{C{{H}_{3}}COON{{H}_{4}}}}\,\xrightarrow{\text{Heat}}\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\xrightarrow{B{{r}_{2}}/KOH}\]
\[\underset{\text{Methyl amine}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,\underset{HCl}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,C{{H}_{3}}OH\underset{300{}^\circ C}{\mathop{\xrightarrow{Cu}}}\,\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
(ii) \[\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\underset{{{H}_{2}}S{{O}_{4}}}{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\xrightarrow{NaOH}\underset{\text{Sod}\text{.acetate}}{\mathop{C{{H}_{3}}COONa}}\,\]
\[\underset{\text{heat}}{\mathop{\xrightarrow{\text{Sodalime}}}}\,\underset{\text{Methane}}{\mathop{C{{H}_{4}}}}\,\underset{hv}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,C{{H}_{3}}Cl\xrightarrow{AgOH}\]
\[C{{H}_{3}}OH\underset{300{}^\circ C}{\mathop{\xrightarrow{Cu}}}\,\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\]
Acetone
It is a symmetrical (simple) ketone and is the first member of the homologous series of ketones. In traces, it is present in blood and urine.
(1) Preparation :
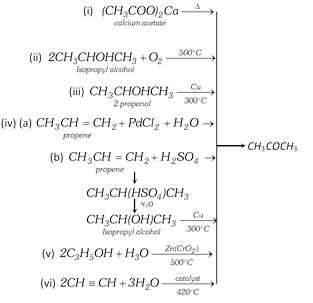
(vii) From pyroligneous acid : Pyroligneous acid containing acetic acid, acetone and methyl alcohol is distilled in copper vessel and the vapours are passed through hot milk of lime. Acetic acid combines to form nonvolatile calcium acetate. The unabsorbed vapours of methanol and acetone are condensed and fractionally distilled. Acetone distills at \[{{56}^{o}}C\].
The acetone thus obtained is purified with the help of sodium bisulphite.
(2) Physical properties : (i) It is a colourless liquid with characteristic pleasant odour.
(ii) It is inflammable liquid. It boils at \[{{56}^{o}}C\].
(iii) It is highly miscible with water, alcohol and ether.
(3) Chemical properties
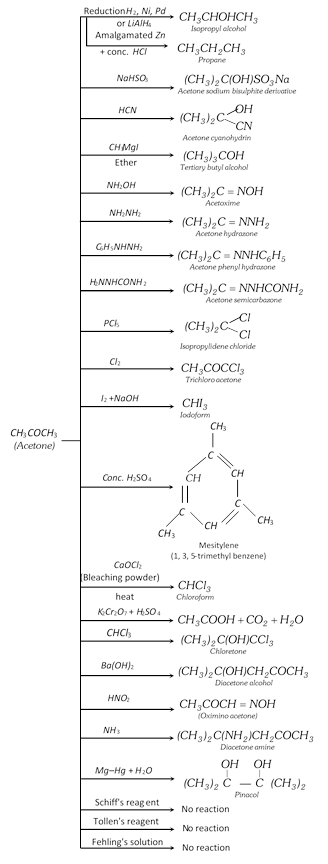
If acetone would be in excess in ketal condensation or catalyst \[(ZnC{{l}_{2}}/\text{dry}\,HCl)\] is used then three moles of acetone undergoes condensation polymerisation and form a compound called ‘Phorone’.
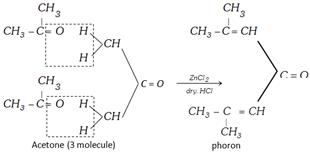
Molecular mass of phorone = 3 mole of acetone – 2 mole of \[{{H}_{2}}O\]
Reformatsky reaction: This reaction involves the treatment of aldehyde and ketone with a bromo acid ester in presence of metallic zinc to form \[\beta -\]hydroxy ester, which can be easily dehydrated into \[\alpha ,\,\,\beta -\]unsaturated ester.
(a)\[BrC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}+Zn\xrightarrow{\text{Benzene}}\underset{\text{Organo zinc compound}}{\mathop{Br-\overset{\oplus }{\mathop{Zn}}\,-C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}}}\,\]
(b) Addition to carbonyl group
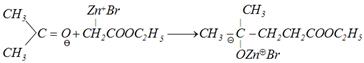
\[\underset{\left[ Zn\,\,\,\,\begin{matrix} Br\ \, \\ OH \\ \end{matrix} \right]}{\mathop{\xrightarrow{HOH/{{H}^{+}}}}}\,\underset{\beta \text{-hydroxyesters}}{\mathop{C{{H}_{3}}-\underset{OH\,\,\,}{\overset{C{{H}_{3}}\,}{\mathop{\underset{|}{\overset{|}{\mathop{{{C}_{{}}}}}}\,\,\,\,-\,\,}}}\,\underset{COO{{C}_{2}}{{H}_{5}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,\,\,\,\,\,\,\,\,\,\,\,\,}}\,}}\,\]
\[\xrightarrow{{}}C{{H}_{3}}-\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C\,\,=\,}}\,}}\,CH-COO{{C}_{2}}{{H}_{5}}\]
(4) Uses
(i) As a solvent for cellulose acetate, cellulose nitrate, celluloid, lacquers, resins, etc.
(ii) For storing acetylene.
(iii) In the manufacture of cordite – a smoke less powder explosive.
(iv) In the preparation of chloroform, iodoform, sulphonal and chloretone.
(v) As a nailpolish remover.
(vi) In the preparation of an artificial scent (ionone), plexiglass (unbreakable glass) and synthetic rubber.
(5) Tests
(i) Legal's test : When a few drops of freshly prepared sodium nitroprusside and sodium hydroxide solution are added to an aqueous solution of acetone, a wine colour is obtained which changes to yellow on standing.
(ii) Indigo test : A small amount of orthonitrobenzaldehyde is added to about 2 ml. of acetone and it is diluted with KOH solution and stirred. A blue colour of indigotin is produced.
(iii) Iodoform test : Acetone gives iodoform test with iodine and sodium hydroxide or iodine and ammonium hydroxide.
Comparison Between Acetaldehyde And Acetone
| Reaction | Acetaldehyde | Acetone |
| Similarities | ||
|
1. Reduction with \[{{H}_{2}}\] and Ni or \[LiAl{{H}_{4}}\] |
Forms ethyl alcohol \[C{{H}_{3}}CHO+{{H}_{2}}\xrightarrow{Ni}C{{H}_{3}}C{{H}_{2}}OH\] |
Forms isopropyl alcohol \[C{{H}_{3}}COC{{H}_{3}}+{{H}_{2}}\xrightarrow{{}}C{{H}_{3}}CHOHC{{H}_{3}}\] |
|
2. Clemmensen's reduction (Zn/Hg and conc. HCl) |
Forms ethane (an alkane) \[C{{H}_{3}}CHO+4H\xrightarrow{{}}C{{H}_{3}}C{{H}_{3}}+{{H}_{2}}O\] |
Forms propane (an alkane) \[C{{H}_{3}}COC{{H}_{3}}+4H\xrightarrow{{}}C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}+{{H}_{2}}O\] |
| 3. Addition of HCN | Forms acetaldehyde cyanohydrin |
Forms acetone cyanohydrin
|
| 4. Addition of \[NaHS{{O}_{3}}\] |
White crystalline derivative
|
White crystalline derivative
|
| 5. Grignard reagent followed by hydrolysis | Forms isopropyl alcohol \[C{{H}_{3}}CHO+C{{H}_{3}}MgI\xrightarrow{{}}{{(C{{H}_{3}})}_{2}}CH-OMgI\]\[\xrightarrow{{{H}_{2}}O}C{{H}_{3}}CHOHC{{H}_{3}}\] | Forms tertiary butyl alcohol \[{{(C{{H}_{3}})}_{2}}CO+C{{H}_{3}}MgI\xrightarrow{{}}{{(C{{H}_{3}})}_{3}}COMgI\] \[\xrightarrow{{{H}_{2}}O}\,{{(C{{H}_{3}})}_{3}}COH\] |
| 6. With hydroxylamine \[(N{{H}_{2}}OH)\] | Forms acetaldoxime (an oxime) \[C{{H}_{3}}CHO+{{H}_{2}}NOH\xrightarrow{{}}C{{H}_{3}}CH=NOH\] | Forms acetoxime (an oxime) \[{{(C{{H}_{3}})}_{2}}CO+{{H}_{2}}NOH\,\,\,\xrightarrow{{}}\,{{(C{{H}_{3}})}_{2}}C=NOH\] |
| 7. With hydrazine \[(N{{H}_{2}}N{{H}_{2}})\] | Forms acetaldehyde hydrazone \[C{{H}_{3}}CHO+{{H}_{2}}NN{{H}_{2}}\xrightarrow{{}}C{{H}_{3}}CH=NN{{H}_{2}}\] | Forms acetone hydrazone \[{{(C{{H}_{3}})}_{2}}CO+{{H}_{2}}NN{{H}_{2}}\xrightarrow{{}}\,{{(C{{H}_{3}})}_{2}}C=NN{{H}_{2}}\] |
| 8. With phenyl hydrazine \[({{C}_{6}}{{H}_{5}}NHN{{H}_{2}})\] | Forms acetaldehyde phenylhydrazone \[C{{H}_{3}}CHO+{{H}_{2}}NNH{{C}_{6}}{{H}_{5}}\xrightarrow{{}}\] \[C{{H}_{3}}CH=NNH{{C}_{6}}{{H}_{5}}\] | Forms acetone phenyl hydrazone \[{{(C{{H}_{3}})}_{2}}CO+{{H}_{2}}NNH{{C}_{6}}{{H}_{5}}\xrightarrow{{}}\] \[{{(C{{H}_{3}})}_{2}}C=NNH{{C}_{6}}{{H}_{5}}\] |
| 9. With semicarbazide \[({{H}_{2}}NNHCON{{H}_{2}})\] | Forms acetaldehyde semicarbazone \[C{{H}_{3}}CHO+{{H}_{2}}NNHCON{{H}_{2}}\xrightarrow{{}}\] \[C{{H}_{3}}CH=NNHCON{{H}_{2}}\] | Forms acetone semicarbazone \[{{(C{{H}_{3}})}_{2}}CO+{{H}_{2}}NNHCON{{H}_{2}}\xrightarrow{{}}\] \[{{(C{{H}_{3}})}_{2}}C=NNHCON{{H}_{2}}\] |
| 10. With \[PC{{l}_{5}}\] | Forms ethylidene chloride (Gem dihalide)  |
Forms isopropylidene chloride (Gem dihalide)
|
| 11. With chlorine | Forms chloral (Gem trihalide) \[C{{H}_{3}}CHO+C{{l}_{2}}\xrightarrow{{}}CC{{l}_{3}}CHO\] | Forms trichloro acetone (Gem trihalide) \[C{{H}_{3}}COC{{H}_{3}}+C{{l}_{2}}\xrightarrow{{}}CC{{l}_{3}}COC{{H}_{3}}\] |
| 12. With alcohols |
Forms acetal (a diether)
|
Forms ketal (a diether)
|
| 13. With \[Se{{O}_{2}}\] |
Forms glyoxal \[C{{H}_{3}}CHO+Se{{O}_{2}}\xrightarrow{{}}\,CHOCHO+Se+{{H}_{2}}O\] |
Forms methyl glyoxal \[{{(C{{H}_{3}})}_{2}}CO+Se{{O}_{2}}\xrightarrow{{}}C{{H}_{3}}COCHO+Se+{{H}_{2}}O\] |
|
14. Iodoform reaction \[({{I}_{2}}+NaOH)\] |
Forms iodoform | Forms iodoform |
| 15. Bleaching powder | Forms chloroform | Forms chloroform |
| 16. Aldol condensation with mild alkali |
Forms aldol \[2C{{H}_{3}}CHO\xrightarrow{{}}C{{H}_{3}}CHOHC{{H}_{2}}CHO\] |
Forms diacetone alcohol \[2C{{H}_{3}}COC{{H}_{3}}\xrightarrow{{}}{{(C{{H}_{3}})}_{2}}C(OH)C{{H}_{2}}COC{{H}_{3}}\] |
| 17. Polymerisation | Undergoes polymerisation | Does not undergo polymerisation but gives condensation reaction |
|
18. With \[N{{H}_{3}}\] |
Forms acetaldehyde ammonia
|
Forms diacetone ammonia \[{{(C{{H}_{3}})}_{2}}CO+N{{H}_{3}}+OC{{(C{{H}_{3}})}_{2}}\xrightarrow{{}}\] \[{{(C{{H}_{3}})}_{2}}C(N{{H}_{2}})C{{H}_{2}}COC{{H}_{3}}\] |
|
19. With conc. \[NaOH\] |
Forms brownish resinous mass | No reaction |
|
20. With \[HN{{O}_{2}}\] |
No reaction |
Forms oximino acetone \[C{{H}_{3}}COC{{H}_{3}}+HN{{O}_{2}}\xrightarrow{{}}C{{H}_{3}}COCH=NOH\] |
| 21. With chloroform | No reaction |
Forms chloretone
|
| 22. With alk. sodium nitroprusside | Deep red colour | Red colour changes to yellow on standing |
| 23. With sodium nitroprusside + Pyridine | Blue colour | No effect |
| 24. Boiling point | \[{{21}^{o}}C\] | \[{{56}^{o}}C\] |
| Dissimilarities | ||
| 25. With Schiff's reagent | Pink colour | Does not give pink colour |
| 26. With Fehling's solution | Gives red precipitate | No reaction |
| 27. With Tollen's reagent | Gives silver mirror | No reaction |
|
28. Oxidation with acidified \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] |
Easily oxidised to acetic acid \[C{{H}_{3}}CHO+O\xrightarrow{{}}C{{H}_{3}}COOH\] |
Oxidation occurs with difficulty to form acetic acid \[C{{H}_{3}}COC{{H}_{3}}+O\xrightarrow{{}}C{{H}_{3}}COOH+C{{O}_{2}}+{{H}_{2}}O\]. |
You need to login to perform this action.
You will be redirected in
3 sec
