Phenol (Carbolic acid), \[{{C}_{6}}{{H}_{5}}OH\] or Hydroxy benzene
Category : JEE Main & Advanced
It was discovered by Runge in the middle oil fraction of coal-tar distillation and named it ‘carbolic acid’ (carbo = coal, oleum = oil) or phenol containing 5% water is liquid at room temperature and is termed as carbolic acid. It is also present in traces in human urine.
(1) Preparation
(i) From benzene sulphonic acid
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,\xrightarrow{{{H}_{2}}S{{O}_{4}}\,(\text{fuming)}}\underset{\text{Benzene sulphonic acid}}{\mathop{{{C}_{6}}{{H}_{5}}S{{O}_{3}}H}}\,\xrightarrow{NaOH}\]
\[\underset{\text{Sodium benzene sulphonate}}{\mathop{{{C}_{6}}{{H}_{5}}S{{O}_{3}}Na}}\,\underset{\text{Fuse}}{\mathop{\xrightarrow{NaOH}}}\,\underset{\text{Sodium phenoxide}}{\mathop{{{C}_{6}}{{H}_{5}}ONa}}\,\underset{\text{or }C{{O}_{2}}/{{H}_{2}}O}{\mathop{\xrightarrow{{{H}^{+}}/{{H}_{2}}O}}}\,\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\].
This is one of the laboratory methods for the preparation of phenol. Similarly methyl phenols (cresols) can be prepared.
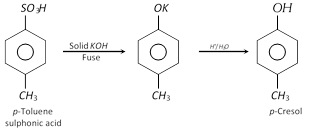
(ii) From benzene diazonium chloride
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,\underset{{{H}_{2}}S{{O}_{4}},\,{{45}^{o}}C}{\mathop{\xrightarrow{HN{{O}_{3}}}}}\,\underset{\text{Nitrobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}N{{O}_{2}}}}\,\xrightarrow{Sn/HCl}\underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,\]
\[\underset{HCl,\,0-{{5}^{o}}C}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\underset{\text{Benzene}\,\text{diazonium}\,\text{chloride}}{\mathop{{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl}}\,\underset{\text{Warm}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\]
(iii) From Grignard reagent
\[\underset{\text{Bromobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Br}}\,+Mg\xrightarrow{\text{Ether}}\underset{\text{Phenyl magnesium bromide}}{\mathop{{{C}_{6}}{{H}_{5}}MgBr}}\,\] \[\xrightarrow{{{O}_{2}}}{{C}_{6}}{{H}_{5}}OMgBr\underset{{{H}^{+}}}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\]
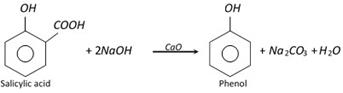
(iv) From salicylic acid :
(v) Middle oil of coal tar distillation : Middle oil of coal-tar distillation has naphthalene and phenolic compounds. Phenolic compounds are isolated in following steps.
Step I : Middle oil is washed with \[{{H}_{2}}S{{O}_{4}}\]. It dissolves basic impurities like pyridine (base).
Step II : Ecessive cooling separates naphthalene (a low melting solid)
Step III : Filtrate of step II is treated with aqueous NaOH when phenols dissolve as phenoxides. Carbon dioxide is then blown through the solution to liberate phenols.
\[{{C}_{6}}{{H}_{5}}OH+NaOH\to {{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}O\]\[\xrightarrow{C{{O}_{2}},\,{{H}_{2}}O}{{C}_{6}}{{H}_{5}}OH+N{{a}_{2}}C{{O}_{3}}\]
Step IV : Crude phenol (of step III) is subjected to fractional distillation.
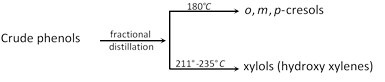
(vi) Raschig’s process
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,+HCl+\frac{1}{2}{{O}_{2}}\underset{{{250}^{o}}C}{\mathop{\xrightarrow{CuC{{l}_{2}}/FeC{{l}_{3}}}}}\,\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+{{H}_{2}}O\]
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+\underset{\text{steam}}{\mathop{{{H}_{2}}O}}\,\xrightarrow{{{425}^{o}}C}\underset{\text{Phenol}}{\mathop{{{C}_{6}}{{H}_{5}}OH}}\,\,\,+HCl\]
(vii) Dow process
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,+2NaOH\underset{\text{High pressure}}{\mathop{\xrightarrow{{{300}^{o}}C}}}\,{{C}_{6}}{{H}_{5}}ONa+NaCl+{{H}_{2}}O\]
sodium phenoxide on treatment with mineral acid yields phenol.
\[2{{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}S{{O}_{4}}\to 2{{C}_{6}}{{H}_{5}}OH+N{{a}_{2}}S{{O}_{4}}\]
(viii) Oxidation of benzene
\[2{{C}_{6}}{{H}_{6}}+{{O}_{2}}\underset{{{315}^{o}}C}{\mathop{\xrightarrow{{{V}_{2}}{{O}_{5}}}}}\,2{{C}_{6}}{{H}_{5}}OH\]
(ix) Oxidation of isopropyl benzene [Cumene]
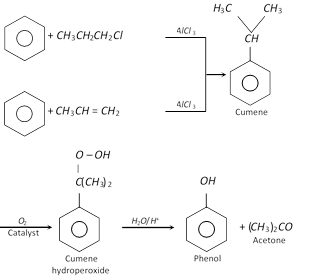
(2) Physical properties
(i) Phenol is a colourless crystalline, deliquescent solid. It attains pink colour on exposure to air and light.
(ii) They are capable of forming intermolecular H-bonding among themselves and with water. Thus, they have high boiling points and they are soluble in water.
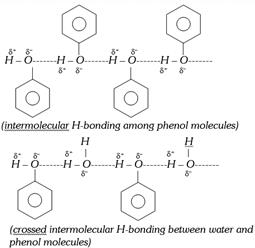
Due to intermolecular H-bonding and high dipole moment, melting points and boiling points of phenol are much higher than that of hydrocarbon of comparable molecular weights.
(iii) It has a peculiar characteristic smell and a strong corrosive action on skin.
(iv) It is sparingly soluble in water but readily soluble in organic solvents such as alcohol, benzene and ether.
(v) It is poisonous in nature but acts as antiseptic and disinfectant.
(3) Chemical properties
(i) Acidic nature : Phenol is a weak acid. The acidic nature of phenol is due to the formation of stable phenoxide ion in solution.
\[{{C}_{6}}{{H}_{5}}OH+{{H}_{2}}O\] ? \[\underset{\text{Phenoxide ion}}{\mathop{{{C}_{6}}{{H}_{5}}{{O}^{-}}}}\,+{{H}_{3}}{{O}^{+}}\]
The phenoxide ion is stable due to resonance.
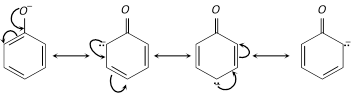
The negative charge is spread throughout the benzene ring. This charge delocalisation is a stabilising factor in the phenoxide ion and increase acidity of phenol. [No resonance is possible in alkoxide ions \[(R{{O}^{-}})\]derived from alcohols. The negative charge is localised on oxygen atom. Thus alcohols are not acidic].
\[{{K}_{a}}(\text{approx}\text{.})\underset{\text{Carboxylic acid}}{\mathop{\underset{RCOOH}{\mathop{({{10}^{-5}})}}\,}}\,>\underset{\text{Carbonic acid}}{\mathop{\underset{{{H}_{2}}C{{O}_{3}}}{\mathop{({{10}^{-7}})}}\,}}\,>\underset{\text{Phenol}}{\mathop{\underset{{{C}_{6}}{{H}_{5}}OH}{\mathop{({{10}^{-10}})}}\,}}\,>\underset{\text{Water}}{\mathop{\underset{HOH}{\mathop{({{10}^{-14}})}}\,}}\,>\underset{\text{Alcohols}}{\mathop{\underset{ROH}{\mathop{({{10}^{-18}})}}\,}}\,\]
Effects of substituents on the acidity of phenols : Presence of electron attracting group, (e.g., \[-N{{O}_{2}}\], \[-X,\] \[-NR_{3}^{+}\], \[-CN,\,\,-CHO,\,\,-COOH\]) on the benzene ring increases the acidity of phenol as it enables the ring to draw more electrons from the phenoxy oxygen and thus releasing easily the proton. Further, the particular effect is more when the substituent is present on o- or p-position than in m-position to the phenolic group.
The relative strengths of some phenols (as acids) are as follows :
p-Nitrophenol > o-Nitrophenol > m- Nitrophenol > Phenol
Presence of electron releasing group, (e.g., \[-C{{H}_{3}}\], \[-{{C}_{2}}{{H}_{5}},\,-OC{{H}_{3}},\,-N{{R}_{2}}\]) on the benzene ring decreases the acidity of phenol as it strengthens the negative charge on phenoxy oxygen and thus proton release becomes difficult. Thus, cresols are less acidic than phenol.
However, m-methoxy and m-aminophenols are stronger acids than phenol because of –I effect and absence of +R effect.
m-methoxy phenol > m-amino phenol > phenol > o-methoxy phenol > p-methoxy phenol
Chloro phenols : o- > m- > p-
Cresols : m- > p- > o-
Dihydric phenol : m- > p- > o-
The acidic nature of phenol is observed in the following :
(a) Phenol changes blue litmus to red.
(b) Highly electropositive metals react with phenol. \[2{{C}_{6}}{{H}_{5}}OH+2Na\to 2{{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}\]
(c) Phenol reacts with strong alkalies to form phenoxides. \[{{C}_{6}}{{H}_{5}}OH+NaOH\to {{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}O\]
However, phenol does not decompose sodium carbonate or sodium bicarbonate, i.e., \[C{{O}_{2}}\] is not evolved because phenol is weaker than carbonic acid.
(ii) Reactions of –OH group
(a) Reaction with \[FeC{{l}_{3}}\]: Phenol gives violet colouration with ferric chloride solution (neutral) due to the formation of a coloured iron complex, which is a characteristic to the existence of keto-enol tautomerism in phenols (predominantly enolic form).
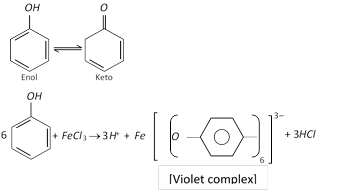
This is the test of phenol.
(b) Ether formation : Phenol reacts with alkyl halides in alkali solution to form phenyl ethers (Williamson’s synthesis). The phenoxide ion is a nucleophile and will replace halogenation of alkyl halide.
\[{{C}_{6}}{{H}_{5}}OH+NaOH\to \underset{\text{Sod}\text{. phenoxide}}{\mathop{{{C}_{6}}{{H}_{5}}ONa}}\,+{{H}_{2}}O\]
\[{{C}_{6}}{{H}_{5}}ONa+ClC{{H}_{3}}\,\,\,\,\,\,\,\to \underset{\text{Methyl phenyl ether (Anisole)}}{\mathop{{{C}_{6}}{{H}_{5}}OC{{H}_{3}}}}\,+\,\,\,\,NaCl\]
\[{{C}_{6}}{{H}_{5}}OK+I{{C}_{2}}{{H}_{5}}\to \underset{\text{Ethoxy benzene (Phenetol)}}{\mathop{{{C}_{6}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}}}\,+KI\]
\[{{C}_{6}}{{H}_{5}}ONa+\underset{\text{Isopropyl chloride}}{\mathop{Cl-HC{{(C{{H}_{3}})}_{2}}}}\,\to \underset{\text{Isopropyl phenyl ether}}{\mathop{{{C}_{6}}{{H}_{5}}-O-HC{{(C{{H}_{3}})}_{2}}}}\,\]
Ethers are also formed when vapours of phenol and an alcohol are heated over thoria \[(Th{{O}_{2}})\] or \[A{{l}_{2}}{{O}_{3}}\].
\[{{C}_{6}}{{H}_{5}}OH+HOC{{H}_{3}}\xrightarrow{\Delta ,\,Th{{O}_{2}}}\underset{\text{Methoxy benzene}}{\mathop{{{C}_{6}}{{H}_{5}}-O-C{{H}_{3}}}}\,\]
(c) Ester formation : Phenol reacts with acid chlorides (or acid anhydrides) in alkali solution to form phenylesters (Acylation). This reaction (Benzoylation) is called Schotten-Baumann reaction.
\[{{C}_{6}}{{H}_{5}}OH+NaOH\to {{C}_{6}}{{H}_{5}}ONa+{{H}_{2}}O\]
\[\underset{\text{Sodium phenoxide}}{\mathop{{{C}_{6}}{{H}_{5}}ONa}}\,+\underset{\text{Acetyl chloride}}{\mathop{Cl\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,C{{H}_{3}}}}\,\to \underset{\text{Phenyl acetate}}{\mathop{{{C}_{6}}{{H}_{5}}OOCC{{H}_{3}}}}\,+NaCl\]
\[{{C}_{6}}{{H}_{5}}OH+\underset{\text{Acetic anhydride}}{\mathop{{{(C{{H}_{3}}CO)}_{2}}O}}\,\xrightarrow{NaOH}\underset{\text{Phenyl acetate (ester)}}{\mathop{{{C}_{6}}{{H}_{5}}OOCC{{H}_{3}}}}\,+C{{H}_{3}}COOH\]\[\underset{\text{Phenyl acetate (ester)}}{\mathop{{{C}_{6}}{{H}_{5}}OOCC{{H}_{3}}}}\,+C{{H}_{3}}COOH\]
\[{{C}_{6}}{{H}_{5}}OH+\underset{\text{Benzoyl chloride}}{\mathop{Cl\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}}}\,\xrightarrow{NaOH}\]\[\underset{\text{Phenyl benzoate}}{\mathop{{{C}_{6}}{{H}_{5}}-O-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}}}\,+NaCl+{{H}_{2}}O\]
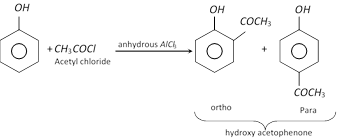
The phenyl esters on treatment with anhydrous \[AlC{{l}_{3}}\] undergoes Fries rearrangement to give o- and p- hydroxy ketones.
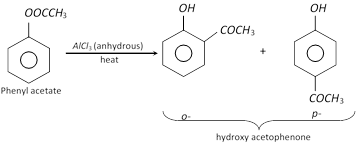
(d) Reaction with \[PC{{l}_{5}}\] : Phenol reacts with \[PC{{l}_{5}}\] to form chlorobenzene. The yield of chlorobenzene is poor and mainly triphenyl phosphate is formed.
\[{{C}_{6}}{{H}_{5}}OH+PC{{l}_{5}}\to {{C}_{6}}{{H}_{5}}Cl+POC{{l}_{3}}+HCl\]
\[3{{C}_{6}}{{H}_{5}}OH+POC{{l}_{3}}\to {{({{C}_{6}}{{H}_{5}})}_{3}}P{{O}_{4}}+3HCl\]
(e) Reaction with zinc dust : When phenol is distilled with zinc dust, benzene is obtained.
\[{{C}_{6}}{{H}_{5}}OH+Zn\to {{C}_{6}}{{H}_{6}}+ZnO\]
(f) Reaction with ammonia : Phenol reacts with ammonia in presence of anhydrous zinc chloride at \[{{300}^{o}}C\] or \[{{(N{{H}_{4}})}_{2}}S{{O}_{3}}/N{{H}_{3}}\] at \[{{150}^{o}}C\] to form aniline. This conversion of phenol into aniline is called Bucherer reaction.
\[{{C}_{6}}{{H}_{5}}OH+N{{H}_{3}}\underset{{{300}^{o}}C}{\mathop{\xrightarrow{ZnC{{l}_{2}}}}}\,\underset{\text{Aniline}}{\mathop{{{C}_{6}}{{H}_{5}}N{{H}_{2}}}}\,+{{H}_{2}}O\]
(g) Action of \[{{P}_{2}}{{S}_{5}}\] : By heating phenol with phosphorus penta sulphide, thiophenols are formed.
\[5{{C}_{6}}{{H}_{5}}OH+{{P}_{2}}{{S}_{5}}\to \underset{\text{Thiophenol}}{\mathop{5{{C}_{6}}{{H}_{5}}SH}}\,+{{P}_{2}}{{O}_{5}}\]
(iii) Reactions of benzene nucleus : The \[-OH\] group is ortho and para directing. It activates the benzene nucleus.
(a) Halogenation : Phenol reacts with bromine in carbon disulphide (or \[CHC{{l}_{3}}\]) at low temperature to form mixture of ortho and para bromophenol.
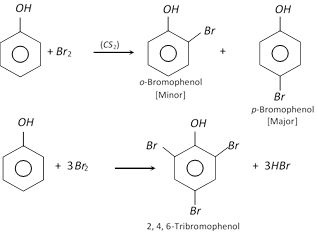
Phenol forms a white precipitate with excess of bromine water yielding 2, 4, 6-tribromophenol.
(b) Sulphonation : Phenol reacts with conc. \[{{H}_{2}}S{{O}_{4}}\] readily to form mixture of ortho and para hydroxy benzene sulphonic acids.
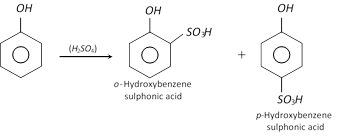
At low temperature \[({{25}^{o}}C)\], the ortho-isomer is the major product, whereas at \[{{100}^{o}}C\], it gives mainly the para-isomer.
(c) Nitration : Phenol reacts with dilute nitric acid at \[5-{{10}^{o}}C\] to form ortho and para nitro phenols, but the yield is poor due to oxidation of phenolic group. The \[-OH\] group is activating group, hence nitration is possible with dilute nitric acid.
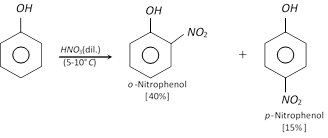
It is believed that the mechanism of the above reaction involves the formation of \[o-\] and \[p-\] nitroso phenol with nitrous acid, \[HN{{O}_{2}}(NaN{{O}_{2}}+HCl)\] at \[0-{{5}^{o}}C,\] which gets oxidised to \[o-\] and \[p-\]nitrophenol with dilute nitric acid.
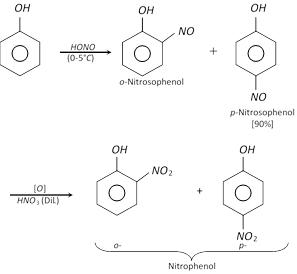
However, when phenol is treated with concentrated \[HN{{O}_{3}}\] in presence of concentrated \[{{H}_{2}}S{{O}_{4}}\], 2,4,6-trinitrophenol (Picric acid) is formed.
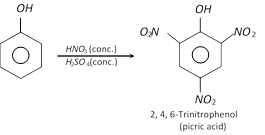
To get better yield of picric acid, first sulphonation of phenol is made and then nitrated. Presence of \[-S{{O}_{3}}H\] group prevents oxidation of phenol.
(d) Friedel-Craft’s reaction : Phenol when treated with methyl chloride in presence of anhydrous aluminium chloride, p-cresol is the main product. A very small amount of o-cresol is also formed.
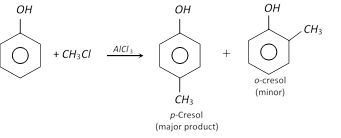
RX and \[AlC{{l}_{3}}\] give poor yields because \[AlC{{l}_{3}}\] coordinates with O. So Ring alkylation takes place as follows,
\[{{C}_{6}}{{H}_{5}}OH+AlC{{l}_{3}}\to {{C}_{6}}{{H}_{5}}OAlC{{l}_{2}}+HCl\]
Thus to carry out successful Friedel-Craft’s reaction with phenol it is necessary to use a large amount of \[AlC{{l}_{3}}\]. The Ring alkylation takes place as follows :
\[{{C}_{6}}{{H}_{5}}OH+\]\[\left\{ \begin{matrix}C{{H}_{3}}CH=C{{H}_{2}} \\{{(C{{H}_{3}})}_{2}}CH-OH\\\end{matrix}\right.\]\[\xrightarrow[or\,\,HF]{{{H}_{2}}S{{O}_{4}}}\] \[o-\] and \[p-\]\[{{C}_{6}}{{H}_{4}}\begin{matrix}\cancel{{}} \\\bcancel{{}} \\\end{matrix}\begin{matrix}OH \\CH{{(C{{H}_{3}})}_{2}}\\\end{matrix}\]
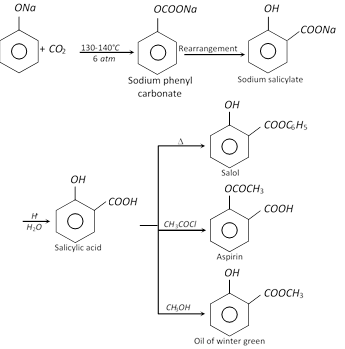
(e) Kolbe-Schmidt reaction (Carbonation) :
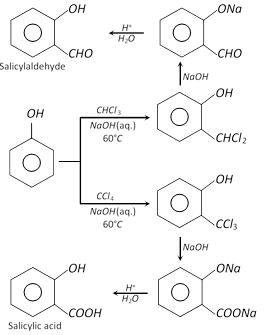
(f) Reimer-Tiemann reaction : Phenol, on refluxing with chloroform and sodium hydroxide (aq.) followed by acid hydrolysis yields salicylaldehyde (o-hydroxy benzaldehyde) and a very small amount of p-hydroxy benzaldehyde. However, when carbon tetrachloride is used, salicylic acid (predominating product) is formed.
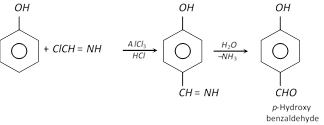
(g) Gattermann’s reaction : Phenol, when treated with liquid hydrogen cyanide and hydrochloric acid gas in presence of anhydrous aluminium chloride yields mainly p-hydroxy benzaldehyde (Formylation).
\[HCl+HC\equiv N\xrightarrow{AlC{{l}_{3}}}ClCH=NH\]
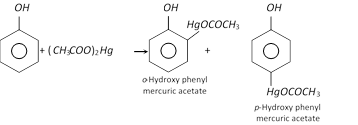
(h) Mercuration
(i) Hydrogenation

(iv) Miscellaneous reactions
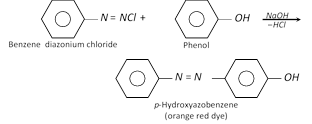
(a) Coupling reactions : Phenol couples with benzene diazonium chloride in presence of an alkaline solution to form a red dye (p-hydroxy azobenzene).
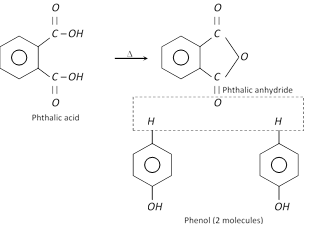
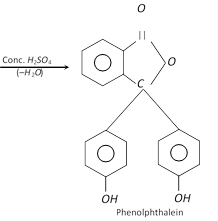
Phenol couples with phthalic anhydride in presence of concentrated \[{{H}_{2}}S{{O}_{4}}\] to form a dye, (phenolphthalein) used as an indicator.
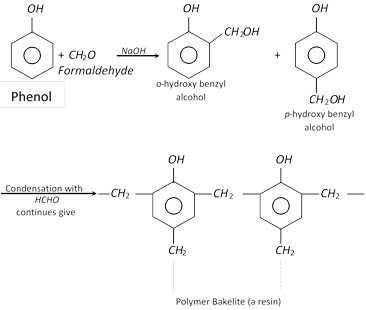
(b) Condensation with formaldehyde : Phenol condenses with formaldehyde (excess) in presence of sodium hydroxide or acid \[({{H}^{+}})\] for about a week to form a polymer known as bakelite (a resin).
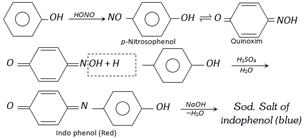
(c) Liebermann’s nitroso reaction : When phenol is reacted with \[NaN{{O}_{2}}\] and concentrated \[{{H}_{2}}S{{O}_{4}}\], it gives a deep green or blue colour which changes to red on dilution with water. When made alkaline with NaOH original green or blue colour is restored. This reaction is known as Liebermann’s nitroso reaction and is used as a test of phenol.
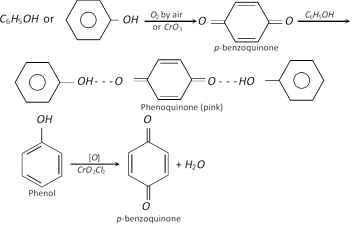
(d) Oxidation : Phenol turns pink or red or brown on exposure to air and light due to slow oxidation. The colour is probably due to the formation of quinone and phenoquinone.
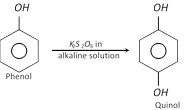
But on oxidation with potassium persulphate in alkaline solution, phenol forms 1, 4-dihydroxy benzene (Quinol). This is known as Elbs persulphate oxidation.
(4) Uses : Phenol is extensively used in industry. The important applications of phenol are
(i) As an antiseptic in soaps, lotions and ointments. A powerful antiseptic is “Dettol” which is a phenol derivative (2, 4-dichloro-3, 5-dimethyl phenol).
(ii) In the manufacture of azo dyes, phenolphthalein, etc.
(iii) In the preparation of picric acid used as an explosive and for dyeing silk and wool.
(iv) In the manufacture of cyclohexanol required for the production of nylon and used as a solvent for rubber and lacquers.
(v) As a preservative for ink.
(vi) In the manufacture of phenol-formaldehyde plastics such as bakelite.
(vii) In the manufacture of drugs like aspirin, salol, phenacetin, etc.
(viii) For causterising wounds caused by the bite of mad dogs.
(ix) As a starting material for the manufacture of nylon and artificial tannins.
(x) In the preparation of disinfectants, fungicides and bactericides.
(5) Tests of phenol
(i) Aqueous solution of phenol gives a violet colouration with a drop of ferric chloride.
(ii) Aqueous solution of phenol gives a white precipitate of 2, 4, 6-tribromophenol with bromine water.
(iii) Phenol gives Liebermann’s nitroso reaction.
Phenol in conc. sulphuric acid \[\underset{\text{Excess of water}}{\mathop{\xrightarrow{NaN{{O}_{2}}}}}\,\] Red colour \[\underset{\text{(Excess)}}{\mathop{\xrightarrow{NaOH}}}\,\] Blue colour
(iv) Phenol combines with phthalic anhydride in presence of conc.\[{{H}_{2}}S{{O}_{4}}\] to form phenolphthalein which gives pink colour with alkali, and used as an indicator.
(v) With ammonia and sodium hypochlorite, phenol gives blue colour.
Difference Between Phenol And Alcohol
| Property | Phenol (C6H5OH) | Alcohol (C2H5OH) |
| Odour | Typical phenolic odour | Pleasant alcoholic odour |
| Nature, reaction with alkali | Acidic, dissolves in sodium hydroxide forming sodium phenoxide. | Neutral, no reaction with alkalies. |
| Reaction with neutral FeCl3 | Gives violet colouration due to formation of complex compound. | No reaction. |
| Reaction with halogen acids | No reaction with halogen acids. | Forms ethyl halides. |
| Oxidation | Pink or brown colour due to formation of quinone and phenoquinone. | Undergoes oxidation to give acetaldehyde and acetic acid. |
| Reaction with HCHO | Forms polymer (bakelite). | No reaction. |
| Liebermann’s nitroso reaction | Positive. | Does not show. |
| Coupling with benzene diazonium chloride | Forms azo dye. | Does not form any dye. |
| Reaction with PCl5 | Mainly forms triphenyl phosphate. | Forms ethyl chloride |
| Iodoform test | Does not show. | Positive. |
You need to login to perform this action.
You will be redirected in
3 sec
