Ether
Category : JEE Main & Advanced
Ethers are anhydride of alcohols, they may be obtained by elimination of a water molecule from two alcohol molecules.
![]()
General formula is \[{{C}_{n}}{{H}_{2n+2}}O\]
General methods of preparation of ethers
(1) From alkyl halides
(i) Williamson?s synthesis
It is a nucleophilic substitution reaction and proceed through \[{{S}_{{{N}^{2}}}}\] mechanism.
\[RONa+{R}'X\to RO{R}'+NaX\]
\[\underset{\text{Sodium ethoxide}}{\mathop{{{C}_{2}}{{H}_{5}}ONa}}\,+C{{H}_{3}}-I\to \underset{\text{Ethyl methyl ether}}{\mathop{C{{H}_{3}}O{{C}_{2}}{{H}_{5}}}}\,+NaI\]
\[\underset{\text{Sodium ethoxide}}{\mathop{{{C}_{2}}{{H}_{5}}ONa}}\,+\underset{\text{Ethyl bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,\to \underset{\text{Ethoxyethane}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+NaBr\]
(a) Order of reactivity of primary halide is \[C{{H}_{3}}X>C{{H}_{3}}C{{H}_{2}}X>C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}X\].
(b) Tendency of alkyl halide to undergo elimination is \[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\].
(c) For better yield alkyl halide should be primary and alkoxide should be secondary or tertiary.
\[\underset{\text{Ethyl bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,+\underset{\begin{smallmatrix} \text{Sodium salt of } \\ \text{tert}\text{. butyl alcohol} \end{smallmatrix}}{\mathop{\underset{\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,\,C{{H}_{3}}}{\mathop{NaO-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,}}\,\to \underset{\text{Ethyl tert}\text{. butyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}-\underset{\,C{{H}_{3}}}{\overset{\,C{{H}_{3}}}{\mathop{O-\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}}\,}}\,\]
(d) Secondary and tertiary alkyl halides readily undergo\[{{E}_{2}}\] elimination in the presence of a strong base to form alkenes.
\[\underset{\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|}{\overset{|}{\mathop{C}}}\,-Cl}}}\,\xrightarrow{{{C}_{2}}{{H}_{5}}ONa}\underset{\,\,\,\,\,C{{H}_{3}}}{\overset{\,\,\,\,\,C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|\,\,\,\,\,\,}{\overset{|\,\,\,\,\,\,}{\mathop{{{C}^{\oplus }}}}}\,+C{{l}^{-}}}}}\,\],
\[\underset{C{{H}_{2}}-H}{\overset{C{{H}_{3}}\,\,\,\,\,\,}{\mathop{C{{H}_{3}}-\underset{|\,\,\,\,\,}{\overset{|\,\,\,\,\,}{\mathop{{{C}^{\oplus }}}}}\,+{{C}_{2}}{{H}_{5}}{{O}^{-}}}}}\,\to \underset{C{{H}_{2}}\,\,\,}{\overset{C{{H}_{3}}\,\,\,}{\mathop{C{{H}_{3}}-\underset{||}{\overset{|}{\mathop{C}}}\,+{{C}_{2}}{{H}_{5}}OH}}}\,\]
(ii) By heating alkyl halide with dry silver oxide
\[2RX+A{{g}_{2}}O\xrightarrow{\text{heat}}R-O-R+2AgX\],
\[\underset{\text{Ethyl bromide}}{\mathop{2{{C}_{2}}{{H}_{5}}Br}}\,+A{{g}_{2}}O\xrightarrow{\text{heat}}\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+2AgBr\]
(2) From alcohols
(i) By dehydration of alcohols
(a) With conc. \[{{H}_{2}}S{{O}_{4}}\] at \[{{140}^{o}}C\]
\[\underset{\text{2 molecules of alcohol}}{\mathop{ROH+HOR}}\,\underset{{{140}^{o}}C}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}(\text{conc}\text{.})}}}\,\underset{\text{Ether}}{\mathop{ROR}}\,+{{H}_{2}}O\].
(b) With \[A{{l}_{2}}{{O}_{3}}\] at \[{{250}^{o}}C\] :
\[2ROH\underset{{{250}^{o}}C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,R-O-R+{{H}_{2}}O\]
(ii) By the action of diazomethane on alcohols : This reaction is in presence of catalyst, boron trifluoride or \[HB{{F}_{4}}\].
\[ROH+C{{H}_{2}}{{N}_{2}}\xrightarrow{B{{F}_{3}}}R-O-C{{H}_{3}}+{{N}_{2}}\]
(a) This method is very useful for preparing mixed ethers.
(b) In higher cases, there can be 1, 2-hydride or 1, 2-methyl shift to form more stable carbonium ion.
(3) Alkoxy mercuration-demercuration
\[>\underset{\text{alkene}}{\mathop{C=C}}\,<+R-OH+\underset{\text{Mercuric trifluoro acetate}}{\mathop{Hg{{[OOCC{{F}_{3}}]}_{2}}}}\,\]
\[\to -\underset{\,OR}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,\,}}\,-\underset{HgOOCC{{F}_{3}}}{\mathop{\,\underset{|}{\overset{|}{\mathop{C}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}\,\xrightarrow{NaB{{H}_{4}}}\underset{\text{Ether}}{\mathop{\underset{\,\,\,\,OR\,\,H}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,\,-\underset{|}{\overset{|}{\mathop{C}}}\,}}\,}}\,\]
(4) Reaction of lower halogenated ether with grignard reagent
\[\underset{\begin{smallmatrix}\text{Halogenated} \\\text{ ether}\end{smallmatrix}}{\mathop{ROC{{H}_{2}}X}}\,+\underset{\begin{smallmatrix}\text{Grignard} \\\text{reagant}\end{smallmatrix}}{\mathop{XMg{R}'}}\,\to \underset{\begin{smallmatrix}\text{Higher } \\\text{ ether}\end{smallmatrix}}{\mathop{ROC{{H}_{2}}{R}'}}\,+Mg{{X}_{2}}\]
(i) Higher members can be prepared by the action of grignard reagent on lower halogenated ethers.
(ii) Ether form soluble coordinated complexes with grignard reagent.
Physical properties
(1) Physical state : Methoxy methane and methoxy ethane are gases while other members are volatile liquid with pleasant smell.
(2) Dipole moment (D.M.) : Bond angle of ether is due to \[s{{p}^{3}}\] hybridisation of oxygen atom. Since C – O bond is a polar bond, hence ether possess a net dipole moment, even if they are symmetrical. dipole moment of dimethyl ether is 1.3 D and dipole moment of di ethyl ether is 1.18 D.
(3) Boiling points : Boiling points of ethers are much lower than those of isomeric alcohols, but closer to alkanes having comparable mass. This is due to the absence of hydrogen bonding in ethers.
(4) Solubility : Solubilities of ethers in water are comparable with those of alcohols.
Example : Di ethyl ether and n-butyl alcohol have approximately the same solubility in water. This is because, ether form hydrogen bond with water much in the same way as alcohol do with water.
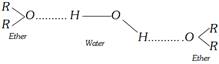
(5) Hydrogen bonding : There is no hydrogen directly attach (bonded) to oxygen in ethers, so ethers do not show any intermolecular hydrogen bonding.
\[\underset{\text{hydrogenbonding in alcohols}}{\mathop{H-\overset{R}{\mathop{\overset{|}{\mathop{O}}\,}}\,\text{---}H-\overset{R}{\mathop{\overset{|}{\mathop{O}}\,}}\,\text{---}H-\overset{R}{\mathop{\overset{|}{\mathop{O}}\,}}\,\text{---}}}\,\] \[\underset{\text{No hydrogen bond in ether}}{\mathop{R-O-R}}\,\]
(6) Density : Ethers are lighter than water.
Chemical properties : Ethers are quite stable compounds. These are not easily attacked by alkalies, dilute mineral acids, active metals, reducing agents or oxidising agents under ordinary conditions.
(1) Reaction due to alkyl group
(i) Halogenation :
\[\underset{\text{Diethyl ether}}{\mathop{C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}}}\,\underset{\text{dark}}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{(\alpha \text{-Monochlorodiethyl ether})}{\mathop{C{{H}_{3}}CHClOC{{H}_{2}}C{{H}_{3}}}}\,\]
\[\underset{\text{Diethyl ether}}{\mathop{C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}}}\,\underset{\text{dark}}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{(\alpha ,\,{\alpha }'\text{-Dichlorodiethyl ether})}{\mathop{C{{H}_{3}}CHClOCHClC{{H}_{3}}}}\,\]
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}+10C{{l}_{2}}\underset{\text{light}}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{(\text{Perchlorodiethyl ether})}{\mathop{{{C}_{2}}C{{l}_{5}}O{{C}_{2}}C{{l}_{5}}}}\,+10\,HCl\]
(ii) Burning : Ethers are highly inflammable. They burn like alkanes.
\[{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}+6{{O}_{2}}\to 4C{{O}_{2}}+5{{H}_{2}}O\]
(2) Reaction due to ethernal oxygen
(i) Peroxide formation :
\[{{C}_{2}}{{H}_{5}}\underset{.\,.}{\overset{.\,.}{\mathop{O}}}\,{{C}_{2}}{{H}_{5}}+\underset{.\,.}{\overset{.\,.}{\mathop{O}}}\,:\,\to \] \[{{({{C}_{2}}{{H}_{5}})}_{2}}O\to O\].
(a) The boiling point of peroxide is higher than that of ether. It is left as residue in the distillation of ether and may cause explosion. Therefore ether may never be evaporated to dryness.
(b) Absolute ether can be prepared by distillation of ordinary ether from conc.\[{{H}_{2}}S{{O}_{4}}\] and subsequent storing over metallic sodium.
\[C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}\xrightarrow{2[O]}\underset{\text{Acetaldehyde}}{\mathop{2C{{H}_{3}}CHO}}\,+{{H}_{2}}O\]
\[\text{Peroxide }+\text{ }F{{e}^{+2}}\to F{{e}^{+3}}\xrightarrow{SC{{N}^{-}}}\underset{\begin{smallmatrix} \text{Blood red colour} \\\text{ (}n=1\text{ to }6\text{)}\end{smallmatrix}}{\mathop{{{[Fe{{(SCN)}_{n}}]}^{3-n}}}}\,\]
(ii) Oxidation with \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{\oplus }}\]
![]()
(a) Oxidation of ether can only be possible if any one of the alkyl groups of ether has hydrogen on \[\alpha -\]carbon.
(b) \[\alpha -\]carbon having two hydrogens converts in carboxylic group and a-carbon having only one hydrogen converts into keto group.
\[C{{H}_{3}}-\overset{\alpha \,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,-O-\overset{{\alpha }'\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,-C{{H}_{2}}-C{{H}_{3}}\]
\[\underset{{{H}^{\oplus }}/\Delta }{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,C{{H}_{3}}-COOH+C{{H}_{3}}-C{{H}_{2}}-COOH\]
\[C{{H}_{3}}-C{{H}_{2}}-O-CH\ \ \ \ \begin{matrix}C{{H}_{3}} \\C{{H}_{3}} \\\end{matrix}\underset{{{H}^{\oplus }}/\Delta }{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,C{{H}_{3}}-COOH+C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\]
\[\underset{{{H}^{\oplus }}/\Delta }{\mathop{\xrightarrow{{{K}_{2}}C{{r}_{2}}{{O}_{7}}}}}\,C{{H}_{3}}-COOH+C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}\]
(iii) Salt formation : Due to lone pair of electrons on oxygen atom. Ether behaves as Lewis base and form stable oxonium salt with strong inorganic acids at low temperature.
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}+HCl\to \underset{\text{Diethyl oxonium chloride}}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{2}}\overset{H\,\,\,}{\mathop{\overset{|\,\,\,\,\,}{\mathop{{{\underset{.\,.}{\mathop{O}}\,}^{+}}}}\,}}\,C{{l}^{-}}}}\,\]
or \[{{[{{({{C}_{2}}{{H}_{5}})}_{2}}O\cdot H]}^{+}}C{{l}^{-}}\]
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}+{{H}_{2}}S{{O}_{4}}\to \underset{\text{Diethyl oxonium hydrogen sulphate}}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{2}}\underset{H}{\overset{.\,.}{\mathop{\underset{|}{\overset{+}{\mathop{O}}}\,}}}\,+HSO_{4}^{-}}}\,\]
or \[{{[{{({{C}_{2}}{{H}_{5}})}_{2}}O\cdot H]}^{+}}HSO_{4}^{-}\]
The oxonium salts are soluble in acid solution and ethers can be recovered from the oxonium salts by treatment with water.
\[\underset{\text{Oxonium salt}}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{2}}\underset{H}{\mathop{\underset{|}{\mathop{O}}\,}}\,Cl}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Diethyl ether}}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{2}}O}}\,+HCl\]
(iv) Reaction with Lewis acids : Being Lewis bases, ethers form complexes with Lewis acids such as \[B{{F}_{3}}\], \[AlC{{l}_{3}}\], \[FeC{{l}_{3}}\], etc. These complexes are called etherates.
![]()
Similarly, diethyl ether reacts with Grignard reagent forming Grignard reagent etherate.
![]()
Due to the formation of the etherate, Grignard reagents dissolve in ether. That is why Grignard reagents are usually prepared in ethers. However, they cannot be prepared in benzene, because benzene has no lone pair of electrons and therefore, cannot form complexes with them.
(3) Reaction involving cleavage of carbon-oxygen bond
(i) Hydrolysis
(a) With dil.\[{{H}_{2}}S{{O}_{4}}\] : \[ROR+{{H}_{2}}O\xrightarrow{{{H}_{2}}S{{O}_{4}}}2ROH\]
\[\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+{{H}_{2}}O\xrightarrow{{{H}_{2}}S{{O}_{4}}}\underset{\text{Ethanol}}{\mathop{2{{C}_{2}}{{H}_{5}}OH}}\,\]
(b) With conc.\[{{H}_{2}}S{{O}_{4}}\] :
\[\underset{\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+2{{H}_{2}}S{{O}_{4}}\to \underset{\text{Ethyl hydrogen sulphate}}{\mathop{2{{C}_{2}}{{H}_{5}}HS{{O}_{4}}}}\,+{{H}_{2}}O}{\mathop{\underline{\underset{{{C}_{2}}{{H}_{5}}OH+{{H}_{2}}S{{O}_{4}}\to {{C}_{2}}{{H}_{5}}HS{{O}_{4}}+{{H}_{2}}O}{\mathop{\,\,\,{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}+{{H}_{2}}S{{O}_{4}}\to {{C}_{2}}{{H}_{5}}OH+{{C}_{2}}{{H}_{5}}HS{{O}_{4}}\,\,}}\,}}}\,\]
(ii) Action of hydroiodic acid
(a) With cold HI
\[\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+HI\xrightarrow{\text{Cold}}\underset{\text{Ethyl iodide}}{\mathop{{{C}_{2}}{{H}_{5}}I}}\,+\underset{\text{Ethyl alcohol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\]
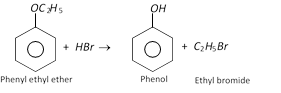
(b) With hot HI
\[R-O-{R}'+2HI\xrightarrow{\text{heat}}RI+{R}'I+{{H}_{2}}O\]
(iii) Zeisel method : \[RI+AgN{{O}_{3}}(\text{alc}\text{.})\to AgI\downarrow +RN{{O}_{3}}\]
(iv) Action of \[PC{{l}_{5}}\]
\[R-O-R+PC{{l}_{5}}\xrightarrow{\text{heat}}2RCl+POC{{l}_{3}}\]. There is no reaction in cold.
(v) Reaction with acetyl chloride
![]()
(vi) Reaction with acid anhydride
\[\underset{\text{Acetic anhydride}}{\mathop{C{{H}_{3}}CO\cdot O\cdot OCC{{H}_{3}}}}\,+\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}\cdot O\cdot {{C}_{2}}{{H}_{5}}}}\,\] \[\underset{\text{heat}}{\mathop{\xrightarrow{ZnC{{l}_{2}}}}}\,\underset{\text{Ethyl acetate}}{\mathop{2C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,\]
(vii) Dehydration
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\underset{{{300}^{o}}C}{\mathop{\xrightarrow{A{{l}_{2}}{{O}_{3}}}}}\,2C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}O\]
(viii) Reaction with carbon mono oxide
\[\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,+CO\underset{500\text{ atm}\text{.}}{\mathop{\xrightarrow{B{{F}_{3}}/{{150}^{o}}C}}}\,\underset{\text{Ethyl propionate}}{\mathop{{{C}_{2}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}}}\,\]
(ix) Action of bases
![]()
\[\to C{{H}_{4}}+C{{H}_{2}}=C{{H}_{2}}+\overset{+}{\mathop{L}}\,i\overset{-}{\mathop{O}}\,{{C}_{2}}{{H}_{5}}\]
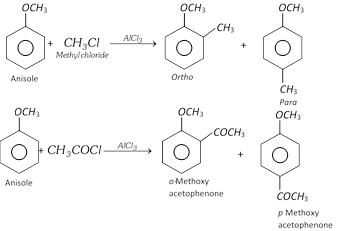
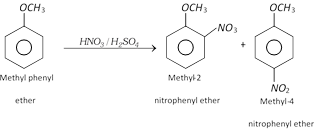
(4) Ring substitution in aromatic ethers : Alkoxy group is ortho and para directing and it directs the incoming groups to ortho and para position. It activates the aromatic ring towards electrophilic substitution reaction.
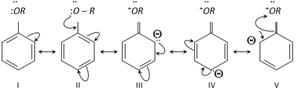
III, IV and V show high electron density at ortho and para position.
(i) Halogenation : Phenyl alkyl ethers undergo usual halogenation in benzene ring.
For example, Bromination of anisole gives ortho and para bromo derivative even in the absence of iron (III) bromide catalyst.
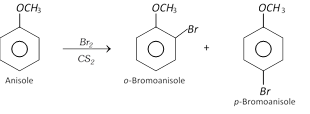
Para isomer is obtained in 90% yield.
(ii) Friedel craft reaction
(iii) Nitration
You need to login to perform this action.
You will be redirected in
3 sec
