Determination of Specific Heat of Liquid
Category : JEE Main & Advanced
If volume, radiating surface area, nature of surface, initial temperature and surrounding of water and given liquid are equal and they are allowed to cool down (by radiation) then rate of loss of heat and fall in temperature of both will be same.
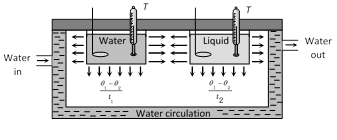
i.e. \[{{\left( \frac{dQ}{dt} \right)}_{\text{water}}}={{\left( \frac{dQ}{dt} \right)}_{\text{liquid}}}\]
\[({{m}_{W}}{{c}_{W}}+W)\frac{({{\theta }_{1}}-{{\theta }_{2}})}{{{t}_{1}}}=({{m}_{l}}{{c}_{l}}+W)\frac{({{\theta }_{1}}-{{\theta }_{2}})}{{{t}_{2}}}\]
or \[\left[ \frac{{{m}_{W}}{{c}_{W}}+W}{{{t}_{1}}} \right]=\left[ \frac{{{m}_{l}}{{c}_{l}}+W}{{{t}_{2}}} \right]\]
\[W={{m}_{c}}{{c}_{c}}=\] Water equivalent of calorimeter, where \[{{m}_{c}}\] and \[{{c}_{c}}\] are mass and specific heat of calorimeter.
If density of water and liquid is \[\rho \] and \[\rho '\] respectively then \[{{m}_{W}}=V{{\rho }_{W}}\] and \[{{m}_{l}}=V\rho {{\,}_{l}}\]
Specific heat of liquid \[{{c}_{l}}=\frac{1}{{{m}_{l}}}\left[ \frac{{{t}_{l}}}{{{t}_{W}}}({{m}_{W}}{{c}_{W}}+W)-W \right]\]
You need to login to perform this action.
You will be redirected in
3 sec
