Spectrum
Category : JEE Main & Advanced
The ordered arrangements of radiations according to wavelengths or frequencies is called Spectrum. Spectrum can be divided in two parts Emission spectrum and Absorption spectrum.
(1) Emission spectrum : When light emitted by a self luminous object is dispersed by a prism to get the spectrum, the spectrum is called emission spectra.
Continuous emission spectrum
(i) It consists of continuously varying wavelengths in a definite wavelength range.
(ii) It is produced by solids, liquids and highly compressed gases heated to high temperature.
(iii) e.g. Light from the sun, filament of incandescent bulb, candle flame etc.

Line emission spectrum
(i) It consist of distinct bright lines.
(ii) It is produced by an excited source in atomic state.
(iii) e.g. Spectrum of excited helium, mercury vapours, sodium vapours or atomic hydrogen.

Band emission spectrum
(i) It consist of district bright bands.
(ii) It is produced by an excited source in molecular state.
(iii) e.g. Spectra of molecular \[{{H}_{2}},\] CO, \[N{{H}_{3}}\] etc.

(2) Absorption spectrum : When white light passes through a semi-transparent solid, or liquid or gas, it's spectrum contains certain dark lines or bands, such spectrum is called absorption spectrum (of the substance through which light is passed).
(i) Substances in atomic state produces line absorption spectra. Polyatomic substances such as \[{{H}_{2}},\]\[C{{O}_{2}}\] and \[KMn{{O}_{4}}\]produces band absorption spectrum.
(ii) Absorption spectra of sodium vapour have two (yellow lines) wavelengths \[{{D}_{1}}(5890\,{\AA})\] and \[{{D}_{2}}(5896\,{\AA})\]
(3) Fraunhoffer's lines : The central part (photosphere) of the sun is very hot and emits all possible wavelengths of the visible light. However, the outer part (chromosphere) consists of vapours of different elements. When the light emitted from the photosphere passes through the chromosphere, certain wavelengths are absorbed. Hence, in the spectrum of sunlight a large number of dark lines are seen called Fraunhoffer lines.
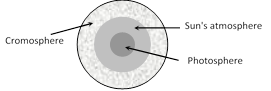
(i) The prominent lines in the yellow part of the visible spectrum were labelled as D-lines, those in blue part as F-lines and in red part as C-line.
(ii) From the study of Fraunhoffer's lines the presence of various elements in the sun's atmosphere can be identified e.g. abundance of hydrogen and helium.
(iii) In the event of a solar eclipse, dark lines become bright. This is because of the reason that the presence of an opaque obstacle in between sun and earth cuts the light off from the central region (photo-sphere), while light from corner portion (cromosphere) is still being received. The bright lines appear exactly at the places where dark lines were present.
(4) Spectrometer : A spectrometer is used for obtaining pure spectrum of a source in laboratory and calculation of \[\mu \] of material of prism and \[\mu \] of a transparent liquid.
It consists of three parts : Collimator which provides a parallel beam of light; Prism Table for holding the prism and Telescope for observing the spectrum and making measurements on it.
The telescope is first set for parallel rays and then collimator is set for parallel rays. When prism is set in minimum deviation position, the spectrum seen is pure spectrum. Angle of prism (A) and angle of minimum deviation \[({{\delta }_{m}})\] are measured and \[\mu \] of material of prism is calculated using prism formula. For \[\mu \] of a transparent liquid, we take a hollow prism with thin glass sides. Fill it with the liquid and measure \[({{\delta }_{m}})\] and A of liquid prism. \[\mu \]of liquid is calculated using prism formula.
(5) Direct vision spectroscope : It is an instrument used to observe pure spectrum. It produces dispersion without deviation with the help of n crown prisms and \[(n-1)\] flint prisms alternately arranged in a tabular structure.
For no deviation \[n\,(\mu -1)A=(n-1)\,\,(\mu \,'-1)A'\].
You need to login to perform this action.
You will be redirected in
3 sec
