Characteristic X-Rays
Category : JEE Main & Advanced
Few of the fast moving electrons having high velocity penetrate the surface atoms of the target material and knock out the tightly bound electrons even from the inner most shells of the atom. Now when the electron is knocked out, a vacancy is created at that place.
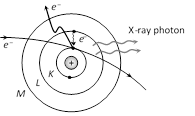
To fill this vacancy electrons from higher shells jump to fill the created vacancies, we know that when an electron jumps from a higher energy orbit
\[{{E}_{1}}\] to lower energy orbit \[{{E}_{2}}\], it radiates energy \[({{E}_{1}}-{{E}_{2}})\]. Thus this energy difference is radiated in the form of X-rays of very small but definite wavelength which depends upon the target material. The X-ray spectrum consists of sharp lines and is called characteristic X-ray spectrum.
(1) K, L, M, ... series : If the electron striking the target eject an electron from the K-shell of the atom, a vacancy is created in the K-shell. Immediately an electron from one of the outer shell, say L-shell jumps to the K-shell, emitting an X-ray photon of energy equal to the energy difference between the two shells. Similarly, if an electron from the M-shell jumps to the K-shell, X-ray photon of higher energy is emitted. The X-ray photons emitted due to the jump of electron from the L, M, N shells to the K-shells gives \[{{K}_{\alpha }},\,\,{{K}_{\beta }},\,\,{{K}_{\gamma }}\] lines of the K-series of the spectrum.
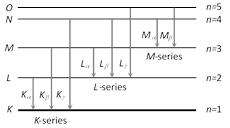
If the electron striking the target ejects an electron from the L-shell of the target atom, an electron from the M, N ... shells jumps to the L-shell so that X-rays photons of lesser energy are emitted.
These photons form the L-series of the spectrum. In a similar way the formation of M series, N series etc. may be explained.
(2) Intensity-wavelength graph : At certain sharply defined wavelengths, the intensity of X-rays is very large as marked \[{{K}_{\alpha }},\,\,{{K}_{\beta }}\] as shown in figure. These X-rays are known as characteristic X-rays. At other wavelengths the intensity varies gradually and these X-rays are called continuous X-rays.

You need to login to perform this action.
You will be redirected in
3 sec
