Nuclear Stability
Category : JEE Main & Advanced
Among about 1500 known nuclides, less than 260 are stable. The others are unstable that decay to form other nuclides by emitting \[\alpha ,\,\,\beta -\]particles and \[\gamma -\]EM waves. (This process is called radioactivity). The stability of nucleus is determined by many factors. Few such factors are given below :
(1) Neutron-proton ratio \[\left( \frac{N}{Z}\,\text{Ratio} \right)\] : The chemical properties of an atom are governed entirely by the number of protons (Z) in the nucleus, the stability of an atom appears to depend on both the number of protons and the number of neutrons.
(i) For lighter nuclei, the greatest stability is achieved when the number of protons and neutrons are approximately equal \[(N\approx Z)\] i.e. \[\frac{N}{Z}=1\]
(ii) Heavy nuclei are stable only when they have more neutrons than protons. Thus heavy nuclei are neutron rich compared to lighter nuclei (for heavy nuclei, more is the number of protons in the nucleus, greater is the electrical repulsive force between them. Therefore more neutrons are added to provide the strong attractive forces necessary to keep the nucleus stable.)
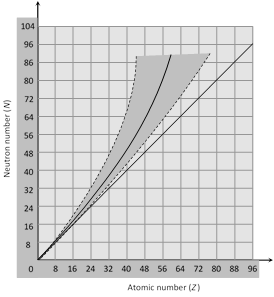
(iii) Figure shows a plot of N verses Z for the stable nuclei. For mass number upto about A = 40. For larger value of Z the nuclear force is unable to hold the nucleus together against the electrical repulsion of the protons unless the number of neutrons exceeds the number of protons. At Bi (Z = 83, A = 209), the neutron excess in \[N-Z=43\]. There are no stable nuclides with \[Z>83\].
(2) Even or odd numbers of Z or N : The stability of a nuclide is also determined by the consideration whether it contains an even or odd number of protons and neutrons.
(i) It is found that an even-even nucleus (even Z and even N) is more stable (60% of stable nuclide have even Z and even N).
(ii) An even-odd nucleus (even Z and odd N) or odd-even nuclide (odd Z and even N) is found to be lesser sable while the odd-odd nucleus is found to be less stable.
(iii) Only five stable odd-odd nuclides are known : \[_{1}{{H}^{2}},\,{{\,}_{3}}L{{i}^{6}},\,{{\,}_{5}}B{{e}^{10}},\,{{\,}_{7}}{{N}^{14}}\,\text{and}{{\text{ }}_{\text{75}}}T{{a}^{180}}\]
(3) Binding energy per nucleon : The stability of a nucleus is determined by value of it's binding energy per nucleon. In general higher the value of binding energy per nucleon, more stable the nucleus is
You need to login to perform this action.
You will be redirected in
3 sec
