Degree of Freedom
Category : JEE Main & Advanced
The term degree of freedom of a system refers to the possible independent motions, systems can have.
or
The total number of independent modes (ways) in which a system can possess energy is called the degree of freedom (f).
The independent motions can be translational, rotational or vibrational or any combination of these.
So the degree of freedom are of three types :
(i) Translational degree of freedom
(ii) Rotational degree of freedom
(iii) Vibrational degree of freedom
General expression for degree of freedom
\[f=3A-B;\] where A = Number of independent particles,
B = Number of independent restriction
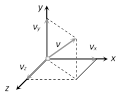
(1) Monoatomic gas : Molecule of monoatomic gas can move in any direction in space so it can have three independent motions and hence 3 degrees of freedom (all translational)
(2) Diatomic gas : Molecules of diatomic gas are made up of two atoms joined rigidly to one another through a bond. This cannot only move bodily, but also rotate about one of the three co-ordinate axes. However its moment of inertia about the axis joining the two atoms is negligible compared to that about the other two axes.
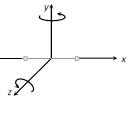
Hence it can have only two rotational motion. Thus a diatomic molecule has 5 degree of freedom : 3 translational and 2 rotational.
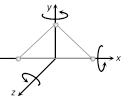
(3) Triatomic gas (Non-linear) : A non-linear molecule can rotate about any of three co-ordinate axes. Hence it has 6 degrees of freedom : 3 translational and 3 rotational.
Degree of freedom for different gases
| Atomicity of gas | Example | A | B |
f = 3 A - B |
Figure |
| Monoatomic | He, Ne, Ar | 1 | 0 | f = 3 | |
| Diatomic | \[{{H}_{2}},{{O}_{2}}{{,}_{{}}}{{N}_{2}},C{{l}_{2}}\] etc. | 2 | 1 | f = 5 | |
| Triatomic non linear | \[{{H}_{2}}O\] | 3 | 3 | f = 6 | 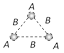 |
| Triatomic linear | \[C{{O}_{2}},BeC{{l}_{2}}\] | 3 | 2 | f = 7 | |
The above degrees of freedom are shown at room temperature. Further at high temperature, in case of diatomic or polyatomic molecules, the atoms with in the molecule may also vibrate with respect to each other. In such cases, the molecule will have an additional degrees of freedom, due to vibrational motion.
An object which vibrates in one dimension has two additional degree of freedom. One for the potential energy and one for the kinetic energy of vibration.
A diatomic molecule that is free to vibrate (in addition to translation and rotation) will have \[7\,(2+3+2)\]degrees of freedom.
You need to login to perform this action.
You will be redirected in
3 sec
