Oxygen Family
Category : JEE Main & Advanced
Oxygen is the first member of group 16 or VIA of the periodic table. It consists of five elements Oxygen (O), sulphur (S), selenium (Se), tellurium (Te) and polonium (Po). These (except polonium) are the ore forming elements and thus called chalcogens.
(1) Electronic configuration
| Elements | Electronic configuration (\[n{{s}^{2}}\ n{{p}^{4}}\]) |
| \[_{8}O\] | \[[He]\,2{{s}^{2}}2{{p}^{4}}\] |
| \[_{16}S\] | \[[Ne]\,3{{s}^{2}}3{{p}^{4}}\] |
| \[_{34}Se\] | \[[Ar]\,3{{d}^{10}}4{{s}^{2}}4{{p}^{4}}\] |
| \[_{52}Te\] | \[[Kr]\,4{{d}^{10}}5{{s}^{2}}5{{p}^{4}}\] |
| \[_{84}Po\] | \[[Xe]\,4{{f}^{14}}5{{d}^{10}}6{{s}^{2}}6{{p}^{4}}\] |
Physical properties
(1) Physical state : Oxygen is gas while all other are solids.
(2) Atomic radii : Down the group atomic radii increases because the increases in the number of inner shells overweighs the increase in nuclear charge.
(3) Ionisaion energy : Down the group the ionisation energy decrease due to increase in their atomic radii and shielding effect.
(4) Electronegativity : Down the group electronegativity decreases due to increase in atomic size.
(5) Electron affinity : Element of this group have high electron affinity, electron affinity decreases down the group.
(6) Non-metallic and metallic character : These have very little metallic character because of their higher ionisation energies.
(7) Nature of bonding : Compound of oxygen with non metals are predominantly covalent. S, Se, and Te because of low electronegativities show more covalent character.
(8) Melting and boiling points : The melting point and boiling points increases on moving down the group.
(9) Catenation : Oxygen has some but sulphur has greater tendency for catenation.
\[\underset{({{H}_{2}}{{O}_{2}})}{\mathop{H-O-O-H,}}\,\,\,\,\,\,\,\underset{({{H}_{2}}{{S}_{2}})}{\mathop{H-S-S-H}}\,,\,\,\,\]
\[\,\,\underset{({{H}_{2}}{{S}_{3}})}{\mathop{H-S-S-S-H}}\,,\,\,\,\,\,\,\underset{({{H}_{2}}{{S}_{4}})}{\mathop{H-S-S-S-S-H}}\,\]
(10) Allotropy
| Oxygen – | \[{{O}_{2}}\] and \[{{O}_{3}}\] |
| Sulphur – | Rhombic , monoclinic, plastic sulphur |
| Selenium – | Red (non-metallic) grey (metallic) |
| Tellurium – | Non-metallic and metallic (more stable) |
| Polonium – | a and b (both metallic) |
(11) Oxidation states : Oxygen shows – 2, + 2 and – 1 oxidation states. Other elements show +2 ,+4 and +6 oxidation states.
Chemical properties
(1) Hydrides : The elements of this group form hydrides such as \[{{H}_{2}}O,\,{{H}_{2}}S,\,{{H}_{2}}Se,\,{{H}_{2}}Te\] an \[{{H}_{2}}Po\]. Following are their characteristics.
(i) Physical states : Water is colourless and odourless while hydrides of the rest of the elements of this group are colourless, unpleasant smelling poisonous gases.
(ii) Volatile nature : Volatility increases from \[{{H}_{2}}O\] to \[{{H}_{2}}S\] and then decreases. The low volatility and abnormally high boiling point of water is due to the association of water molecules on account of hydrogen bonding because of strongly electronegative oxygen atom linked to hydrogen atom. thus, water is liquid while \[{{H}_{2}}S\] and other hydrides are gases under normal condition of temperature and pressure.
(iii) Acidic character : The hydrides of this group behave as weak diprotic acids in aqueous solution, the acidic character increasing from \[{{H}_{2}}S\] to \[{{H}_{2}}Te\] when \[{{H}_{2}}O\] is neutral.
(iv) Thermal stability : The thermal stability decreases from \[{{H}_{2}}O\] to \[{{H}_{2}}Po\] because the size of the central atom (from \[O\] to \[Po\]) increases resulting in longer and weaker \[M-H\] bond consequently the bond strength decreases. This results in the decrease of the thermal stability.
(v) Reducing character : The reducing power of the hydrides increases from \[{{H}_{2}}O\] to \[{{H}_{2}}Po\] due to the decreasing bond strength from \[{{H}_{2}}O\] to \[{{H}_{2}}Po\].
(vi) Bond angle : All these hydrides are angular molecules and the bond angle \[H-X-H\](\[X\] is \[O,\,S,\,Se,\,Te\]) decreases from \[{{H}_{2}}O\] to \[{{H}_{2}}Te\].
Increasing order of reducing power of hydrides :
\[{{H}_{2}}O<{{H}_{2}}S<{{H}_{2}}Se<{{H}_{2}}Te\]
Increasing order of bond angles in hydrides :
\[{{H}_{2}}Te<{{H}_{2}}Se<{{H}_{2}}S<{{H}_{2}}O\]
The order of stability of hydrides :
\[{{H}_{2}}O\ >\ {{H}_{2}}S\ >\ {{H}_{2}}Se\ >\ {{H}_{2}}Te\]
The order of increasing acidic nature of hydrides :
\[{{H}_{2}}O<{{H}_{2}}S<{{H}_{2}}Se<{{H}_{2}}Te\]
(2) Oxides : These elements form monoxides (MO), dioxides (\[M{{O}_{2}}\]) and trioxides \[(M{{O}_{3}})\].
(i) Dioxides : Sulphur, selenium and tellurium burn in air to form \[S{{O}_{2}},\,Se{{O}_{2}}\] and \[Te{{O}_{2}}\]. The dioxide molecules contain \[p\pi -p\pi \] bonds which become weaker with increase in atomic number because of the increase in the bond length.
(a) Sulphur dioxide, \[S{{O}_{2}}\] is a gas at room temperature and exists as individual molecules even in the solid state. Its molecule has bent structure and is a resonance hybrid of the following canonical structures.
![]()
\[S{{O}_{2}}\] is acidic in nature and also called the anhydride of sulphurous acid. It can act as reducing and oxidising agent. \[S{{O}_{2}}\] also acts as a beleaching agent in the presence of moisture, but in contrast to \[C{{l}_{2}}\], its bleaching action is temporary.
\[S{{O}_{2}}+2{{H}_{2}}O\xrightarrow{{}}{{H}_{2}}S{{O}_{4}}+2[H]\]
Colouring matter \[+2[H]\] ? Colourless compound
Hence, \[S{{O}_{2}}\] bleaches due to reduction and the bleaching action is temporary.
(b) Selenium dioxide, \[Se{{O}_{2}}\] is a solid with polymeric zig-zag structure at room temperature however it exist as discrete molecules in the gaseous phase.
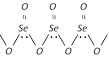
(c) Tellurium dioxide, \[Te{{O}_{2}}\] is also a solid with polymeric zig-zag structure at room temperature very similar to that of selenium dioxide.
(ii) Trioxides : Sulphur, selenium and tellurium can form trioxides also.
(a) Sulphur trioxide, \[S{{O}_{3}}\] : In the gaseous state monomeric \[S{{O}_{3}}\] has a planar structure with \[S-O\] bond distance of 143 pm and \[O-S-O\] bond angle of \[{{120}^{o}}.\,S{{O}_{3}}\] molecule is a resonance hybrid of following structures.
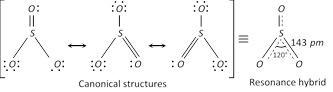
In the solid phase sulphur trioxide polymerises to cyclic trimer or to a stable linear chain structure. \[S{{O}_{3}}\] is the anhydride of \[{{H}_{2}}S{{O}_{4}}\]. It is acidic in nature and acts as oxidising agent.
(b) Selenium trioxide, \[Se{{O}_{3}}\] : it is a solid substance which exists as a cyclic tetramer, however in the vapour phase it exists as a monomer
(c) Tellurium trioxide, \[Te{{O}_{3}}\] : It is a solid at room temperature existing as a polymer.
The increasing order of acidic nature of oxides is \[Te{{O}_{3}}<Se{{O}_{3}}<S{{O}_{3}}\].
(3) Oxyacids : \[{{H}_{2}}S{{O}_{3}},{{H}_{2}}S{{O}_{4}},{{H}_{2}}{{S}_{2}}{{O}_{3}},{{H}_{2}}S{{O}_{5}},{{H}_{2}}{{S}_{2}}{{O}_{8}},{{H}_{2}}{{S}_{2}}{{O}_{7}},{{H}_{2}}{{S}_{2}}{{O}_{6}}\]
(4) Halides : Oxygen : \[O{{F}_{2}},C{{l}_{2}}O,B{{r}_{2}}O\]
Sulphur : \[{{S}_{2}}{{F}_{2}},{{S}_{2}}C{{l}_{2}},S{{F}_{2}},SC{{l}_{2}},SB{{r}_{2}},S{{F}_{4}},SC{{l}_{4}}\] and \[S{{F}_{6}}\]
Selenium and tellurium : \[Se{{F}_{6}}\] and \[Te{{F}_{6}}\]
Anamolous Behaviour of Oxygen
Oxygen is the first member of the group 16 family and differs from the other members of the family because of
(1) Its small size
(2) Its high electronegativity
(3) Its high ionisation energy
(4) Absence of \[d\]-orbitals in the valence shell
It differs from the other members of the family as follows
(1) Elemental state : Oxygen is a diatomic gas while others are octa-atomic solids with eight membered puckered ring structure.
(2) Oxidation states : Oxygen shows O.S. of –2 in most of its compounds. It also shows an O. S. of +2 in \[{{F}_{2}}O\] and –1 in \[{{H}_{2}}{{O}_{2}}\] or other peroxides. It cannot show O.S. beyond 2. Other elements show oxidation states of +2, +4 and +6 because these elements have vacant \[d\]-orbitals so that their valence shell can expand.
(3) Hydrogen-bonding : Oxygen atom is very small and has quite high nuclear charge. therefore, it has high value of electronegativity and is able to form -bonds. the other elements, because of their large size, cannot form -bonds. As a result, is liquid while is a gas and etc., are solids.
(4) Maximum covalency : Oxygen has a maxium covalency of two while other elements can show a maximum covalency of six. This is because these elements have vacant -orbitals while oxygen has not.
(5) Types of compounds : The compounds of oxygen are mainly ionic and polar covalent due to high electronegativity of oxygen while those of others are not.
(6) Magnetic character : Oxygen is paramagnetic while others are not.
Oxygen and its compounds
Oxygen is the most abundant element in the earth crust (46.5%). It was discovered by Karl Scheele and Joseph Priestley. It occurs in three isotopic forms :
\[\underset{(Abundance:\,99.76%)}{\mathop{{}_{8}{{O}^{16}}}}\,\]
\[\underset{(Abundance:\,0.037%)}{\mathop{{}_{8}{{O}^{17}}}}\,\]
\[\underset{(Abundance:\,0.204%)}{\mathop{{}_{8}{{O}^{18}}}}\,\]
Out of the three isotopes, \[{}_{8}{{O}^{18}}\] is radioactive.
Occurrence : In free state, it occurs in air and constitutes 21% by volume of air.
Preparation of Dioxygen : Oxygen is prepared by the following methods.
(1) By the decomposition of oxygen rich compounds : e.g.
\[\underset{Pot.\,Nitrate}{\mathop{2KN{{O}_{3}}}}\,\xrightarrow{Heat}2KN{{O}_{2}}+{{O}_{2}}\];
\[\underset{Pot.\,Chlorate}{\mathop{2KCl{{O}_{3}}}}\,\underset{Mn{{O}_{2}}}{\mathop{\xrightarrow{Heat}}}\,2KCl+3{{O}_{2}}\]
(2) By heating dioxides, Peroxides and higher oxides : e.g.
\[\underset{Silver\,oxide}{\mathop{2A{{g}_{2}}O}}\,\xrightarrow{Heat}4Ag+{{O}_{2}}\];
\[\underset{Manganese\,dioxide}{\mathop{3Mn{{O}_{2}}}}\,\xrightarrow{Heat}M{{n}_{3}}{{O}_{4}}+{{O}_{2}}\]
\[\underset{Barium\,peroxide}{\mathop{2Ba{{O}_{2}}}}\,\xrightarrow{Heat}\underset{Barium\,oxide}{\mathop{2BaO}}\,+{{O}_{2}}\]
(3) Laboratory Method : In the laboratory, is prepared by thermal decomposition of potassium chlorate.
\[2KCl{{O}_{3}}\underset{Mn{{O}_{2}}}{\mathop{\xrightarrow{420\,K}}}\,2KCl+3{{O}_{2}}\]
In the absence of \[Mn{{O}_{2}}\] catalyst, the decomposition takes place at 670-720 K. Therefore,\[Mn{{O}_{2}}\] acts as a catalyst and also lowers the temperature for the decomposition of \[KCl{{O}_{3}}.\]
(4) \[{{O}_{2}}\] can also be prepared by the action of water on sodium peroxide as, \[2N{{a}_{2}}{{O}_{2}}+2{{H}_{2}}O\to 4NaOH+{{O}_{2}}\].
(5) Industrial preparation : The main sources for the industrial preparation of dioxygen are air and water.
(i) From air : \[{{O}_{2}}\] is prepared by fractional distillation of air. During this process, \[{{N}_{2}}\]with less boiling point (78 K) distills as vapour while \[{{O}_{2}}\] with higher boiling point (90 K) remains in the liquid state and can be separated.
(ii) From water : \[{{O}_{2}}\] can also be obtained by the electrolysis of water containing a small amount of acid or alkali,
\[2{{H}_{2}}O2{{H}_{2}}(g)+{{O}_{2}}(g)\]
Physical properties of O2 : It is a colourless, tasteless and odourless gas. It is slightly soluble in water and its solubility is about \[30\,c{{m}^{3}}\,\text{per}\,\text{litre}\] of water at 298 K.
Physical properties of atomic and molecular oxygen
| Atomic properties | Molecular properties |
| Atomic radius (pm) – 73 | Bond length (pm) – 120.7 |
| Ionic radius O2– (pm) – 140 | Bond energy (kJ mol–1) – 493 |
| Electronegativity – 3.5 | Density at S.T.P. (gcm–3)– 1.429 |
| Ionisation energy (kJ mol–1) – 1310 | Melting point (K) – 54.4 |
| Electron affinity (kJ mol–1) – 140 | Boiling point (K) – 90.2 |
Chemical properties of O2 : It does not burn itself but helps in burning. It is quite stable in nature and its bond dissociation energy is very high. Therefore, it is not very reactive as such, \[{{O}_{2}}\to O+O\].
Therefore, dioxygen reacts at higher temperatures. However, once the reaction starts, it proceeds of its own. This is because the chemical reactions of dioxygen are exothermic and the heat produced during the reaction is sufficient to sustain the reactions.
(1) Action with litmus : Like dihydrogen, it is also neutral and has no action on blue or red litmus.
(2) Reaction with metals : Active metals like Na, Ca react at room temp. to form their respective oxides.
\[4Na+{{O}_{2}}\to 2N{{a}_{2}}O\]; \[2Ca+{{O}_{2}}\to 2CaO\]
It reacts with Fe, Al, Cu etc. metals at high temperature
\[4Al+3{{O}_{2}}\to 2A{{l}_{2}}{{O}_{3}}\]; \[4Fe+3{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}\]
(3) Action with Non-metals : It form oxides.
\[2{{H}_{2}}+{{O}_{2}}\overset{1073\,K}{\mathop{\xrightarrow[\text{Electric}\,\text{discharge}]{}}}\,2{{H}_{2}}O\] ;
\[{{N}_{2}}+{{O}_{2}}\xrightarrow{3273\,K}\underset{Nitric\,oxide}{\mathop{2NO}}\,\]
\[S+{{O}_{2}}\xrightarrow{Heat}S{{O}_{2}}\] ; \[C+{{O}_{2}}\xrightarrow{Heat}C{{O}_{2}}\]
(4) Reaction with compounds : Dioxygen is an oxidising agent and it oxidises many compounds under specific conditions. e.g.
\[4HCl+{{O}_{2}}\overset{700\,K}{\mathop{\xrightarrow[CuC{{l}_{2}}]{}}}\,2{{H}_{2}}O+2C{{l}_{2}}\];
\[4N{{H}_{3}}+5{{O}_{2}}\underset{Pt}{\mathop{\xrightarrow{1073\,K}}}\,4NO+6{{H}_{2}}O\]
\[C{{S}_{2}}+3{{O}_{2}}\xrightarrow{Heat}C{{O}_{2}}+2S{{O}_{2}}\];
\[C{{H}_{4}}+2{{O}_{2}}\to C{{O}_{2}}+2{{H}_{2}}O\]
Uses of dioxygen
(1) It is used in the oxy-hydrogen or oxy-acetylene torches which are used for welding and cutting of metals.
(2) It is used as an oxidising and bleaching agent,
(3) Liquid \[{{O}_{2}}\] is used as rocket fuel.
(4) It is used in metallurgical processes to remove the impurities of metals by oxidation.
Compounds of Oxygen
(1) Oxides : A binary compound of oxygen with another element is called oxide. On the basis of acid-base characteristics, the oxides may be classified into the following four types,
(i) Basic oxides : Alkali, alkaline earth and transition metals form basic oxides - \[N{{a}_{2}}O,\,MgO,\,F{{e}_{2}}{{O}_{3}}\] etc. their relative basic character decreases in the order : alkali metal oxides>alkaline earth metal oxides>transition metal oxides.
(ii) Acidic oxides : Non-metal oxides are generally acidic -
\[C{{O}_{2}},S{{O}_{2}},S{{O}_{3}},N{{O}_{2}},\,{{N}_{2}}{{O}_{5}},{{P}_{4}}{{O}_{10}},C{{l}_{2}}{{O}_{7}}\] etc.
(iii) Amphoteric oxides : \[A{{l}_{2}}{{O}_{3}},Sn{{O}_{2}}\]etc.
(iv) Neutral oxides : \[{{H}_{2}}O,\,CO,\,{{N}_{2}}O,\,NO\] etc.
Chemical properties of \[{{O}_{2}}\] : It does not burn itself but helps in burning. It is quite stable in nature and its bond dissociation energy is very high. Therefore, it is not very reactive as such, \[{{O}_{2}}\to O+O\].
Therefore, dioxygen reacts at higher temperatures. However, once the reaction starts, it proceeds of its own. This is because the chemical reactions of dioxygen are exothermic and the heat produced during the reaction is sufficient to sustain the reactions.
(1) Action with litmus : Like dihydrogen, it is also neutral and has no action on blue or red litmus.
(2) Reaction with metals : Active metals like Na, Ca react at room temp. to form their respective oxides.
\[4Na+{{O}_{2}}\to 2N{{a}_{2}}O\]; \[2Ca+{{O}_{2}}\to 2CaO\]
It reacts with Fe, Al, Cu etc. metals at high temperature
\[4Al+3{{O}_{2}}\to 2A{{l}_{2}}{{O}_{3}}\]; \[4Fe+3{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}\]
(3) Action with Non-metals : It form oxides.
\[2{{H}_{2}}+{{O}_{2}}\overset{1073\,K}{\mathop{\xrightarrow[\text{Electric}\,\text{discharge}]{}}}\,2{{H}_{2}}O\] ;
\[{{N}_{2}}+{{O}_{2}}\xrightarrow{3273\,K}\underset{Nitric\,oxide}{\mathop{2NO}}\,\]
\[S+{{O}_{2}}\xrightarrow{Heat}S{{O}_{2}}\] ; \[C+{{O}_{2}}\xrightarrow{Heat}C{{O}_{2}}\]
(4) Reaction with compounds : Dioxygen is an oxidising agent and it oxidises many compounds under specific conditions. e.g.
\[4HCl+{{O}_{2}}\overset{700\,K}{\mathop{\xrightarrow[CuC{{l}_{2}}]{}}}\,2{{H}_{2}}O+2C{{l}_{2}}\];
\[4N{{H}_{3}}+5{{O}_{2}}\underset{Pt}{\mathop{\xrightarrow{1073\,K}}}\,4NO+6{{H}_{2}}O\]
\[C{{S}_{2}}+3{{O}_{2}}\xrightarrow{Heat}C{{O}_{2}}+2S{{O}_{2}}\];
\[C{{H}_{4}}+2{{O}_{2}}\to C{{O}_{2}}+2{{H}_{2}}O\]
Uses of dioxygen
(1) It is used in the oxy-hydrogen or oxy-acetylene torches which are used for welding and cutting of metals.
(2) It is used as an oxidising and bleaching agent,
(3) Liquid \[{{O}_{2}}\] is used as rocket fuel.
(4) It is used in metallurgical processes to remove the impurities of metals by oxidation.
Compounds of Oxygen
(1) Oxides : A binary compound of oxygen with another element is called oxide. On the basis of acid-base characteristics, the oxides may be classified into the following four types,
(i) Basic oxides : Alkali, alkaline earth and transition metals form basic oxides - \[N{{a}_{2}}O,\,MgO,\,F{{e}_{2}}{{O}_{3}}\] etc. their relative basic character decreases in the order : alkali metal oxides>alkaline earth metal oxides>transition metal oxides.
(ii) Acidic oxides : Non-metal oxides are generally acidic -\[C{{O}_{2}},S{{O}_{2}},S{{O}_{3}},N{{O}_{2}},\,{{N}_{2}}{{O}_{5}},{{P}_{4}}{{O}_{10}},C{{l}_{2}}{{O}_{7}}\] etc.
(iii) Amphoteric oxides : \[A{{l}_{2}}{{O}_{3}},Sn{{O}_{2}}\]etc.
(iv) Neutral oxides : \[{{H}_{2}}O,\,CO,\,{{N}_{2}}O,\,NO\] etc.
Trends of oxides in the periodic Table : On moving from left to the right in periodic table, the nature of the oxides change from basic to amphoteric and then to acidic. For example, the oxides of third period has the following behaviour,
|
\[N{{a}_{2}}O\] strongly basic |
\[MgO\] basic |
\[A{{l}_{2}}{{O}_{3}}\] amphoteric |
\[Si{{O}_{2}}\] weakly acidic |
\[{{P}_{4}}{{O}_{10}}\] acidic |
\[S{{O}_{2}}\] strongly acidic |
\[C{{l}_{2}}{{O}_{7}}\]very strongly acidic |
![]()
However, on moving down a group, acidic character of the oxides decreases. For example in the third group, the acidic character of oxides decreases as:
|
\[{{B}_{2}}{{O}_{3}}\] acidic |
\[A{{l}_{2}}{{O}_{3}}\] amphoteric |
\[G{{a}_{2}}{{O}_{3}}\] (weakly basic) |
\[I{{n}_{2}}{{O}_{3}},\,T{{l}_{2}}{{O}_{3}}\] basic |
![]()
On the basis of oxygen content the oxides may be classified into the following types,
Normal oxides : These contain oxygen atoms according to the normal oxidation number i.e. – 2. For example, \[MgO,\] \[\,{{H}_{2}}O,\] \[\,CaO,\,L{{i}_{2}}O,\,A{{l}_{2}}{{O}_{3}}\]etc.
Polyoxides : These contain oxygens atoms more than permitted by the normal valency. Therefore, these contain oxygen atoms in oxidation state different than –2.
Peroxides : These contains \[O_{2}^{2-}\] ion having oxidation number of oxygen as –1. For example,
\[{{H}_{2}}{{O}_{2}},\,N{{a}_{2}}{{O}_{2}},\,Ba{{O}_{2}},\,Pb{{O}_{2}}\]etc.
Superoxides : These contains \[O_{2}^{-}\] ion having oxidation number of oxygen as –1/2. For example, \[K{{O}_{2}},\,Pb{{O}_{2}},\,\]etc.
Suboxides : These oxides contain less oxygen than expected from the normal valency. For example, \[{{N}_{2}}O.\]
Mixed oxides : These oxides are made up of two simple oxides. For example, red lead \[P{{b}_{3}}{{O}_{4}}(2Pb{{O}_{2}}+Pb{{O}_{2}}),\] magnetic oxide of iron, \[F{{e}_{3}}{{O}_{4}}(FeO+F{{e}_{2}}{{O}_{3}})\] and mixed oxide of manganese, \[M{{n}_{3}}{{O}_{4}}(Mn{{O}_{2}}+2MnO).\]
Ozone or trioxygen
Ozone is an allotrope of oxygen. It is present in the upper atmosphere, where it is formed by the action of U. V. radiations on \[{{O}_{2}}\], \[3{{O}_{2}}\xrightarrow{U.V.\,\text{radiation}}\underset{Ozone}{\mathop{2{{O}_{3}}}}\,\].
\[{{O}_{3}}\] protects us from the harmful U. V. radiations which causes skin cancer. Now a days, ozone layer in the atmosphere is depleting due to NO released by supersonic aircrafts and chlorofluoro carbons (CFC’S) i.e. freon which is increasingly being used in aerosols and as a refrigerant.
Preparation : Ozone is prepared by passing silent electric discharge through pure, cold and dry oxygen in a specially designed apparatus called ozoniser. The formation of ozone from oxygen is an endothermic reaction.
\[3{{O}_{3}}2{{O}_{3}}\,\,\,\Delta H=+285.4\,\,kJ\]
Ozone is prepared in the laboratory by the following two types of ozonisers,
(a) Siemen’s ozoniser, (b) Brodie’s ozoniser
For the better yield of ozone : (a) Only pure and dry oxygen should be used. (b) The ozoniser must be perfectly dry. (c) A fairly low temperature \[(\approx 273\,K)\]must be maintained. (d) The electric discharge must be sparkless.
Physical properties : Ozone is a light blue coloured gas, having pungent odour. It is heavier than air. Its vapour density is 24. It is slightly soluble in water.
Chemical properties : The important chemical properties of ozone are discussed below,
(1) Decomposition : Pure ozone decomposes on heating above 475 K to form \[{{O}_{2}}\] gas.
\[\text{ 2}{{\text{O}}_{\text{3}}}\xrightarrow{475\,K}3{{O}_{2}}\] \[\Delta H=-285.4\,kJ\]
(2) Oxidising agent : Ozone is one of the most powerful oxidising agent with the liberation of dioxygen. In fact, ozone is a stronger oxidising agent than molecular oxygen because ozone has higher energy content and decomposes to give atomic oxygen as:
\[{{O}_{3}}\to {{O}_{2}}+\underset{\text{Atomic}\,\text{oxygen}}{\mathop{O}}\,\]
Therefore, ozone oxidises a number of non-metals and other reducing agents. e.g.
\[\underset{Metal}{\mathop{2Ag}}\,+{{O}_{3}}\to \underset{Silver\,oxide}{\mathop{A{{g}_{2}}O}}\,+{{O}_{2}}\];
\[\underset{Non-metal}{\mathop{S}}\,+3{{O}_{3}}\to S{{O}_{3}}+3{{O}_{2}}\]
\[\underset{Compound}{\mathop{PbS}}\,+4{{O}_{3}}\to PbS{{O}_{4}}+4{{O}_{2}}\]
Mercury is oxidised to mercurous oxide,
\[2Hg+{{O}_{3}}\to \underset{Mercurous\,oxide}{\mathop{H{{g}_{2}}O}}\,+{{O}_{2}}\]
During this reaction mercury loses its meniscus and starts sticking to the sides of the glass. This is known as tailing of mercury. Mercurous oxide formed in this reaction dissolves in mercury and starts sticking to the glass surface.
(3) Bleaching agent : Due to the oxidising action of ozone, it acts as a mild bleaching agent as well as a sterilizing agent. It acts as a bleaching agent for vegetable colouring matter.
\[Vegetable\,\ colouring\,\ matter+{{O}_{3}}\to \underset{(Colourless)}{\mathop{Oxidised\,coloured\ matter}}\,+{{O}_{2}}\]
For example, ozone bleaches indigo, ivory, litmus, delicate fabrics etc.
(4) Formation of ozonides : Ozone reacts with alkenes in the presence of \[CC{{l}_{4}}\]to form an ozonide. e.g.
\[\underset{Ethylene}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+{{O}_{3}}\xrightarrow{CC{{l}_{4}}}{{H}_{2}}C\] 
Structure of \[{{O}_{3}}\]: The structure of \[{{O}_{3}}\] molecule is angular as shown in fig. The \[O-O-O\]bond angle is 116.8° and \[O-O\]bond length is 128 pm.

Uses of ozone
(1) \[{{O}_{3}}\]is used for disinfecting water for drinking purposes because ozone has germicidal properties.
(2) It is used for purifying air of crowded places such as cinemas, under ground railway, auditoriums, tunnels, mines etc.
(3) It is used in industry for the manufacture of \[KMn{{O}_{4}},\] artificial silk, synthetic camphor etc.
Sulphur and its compounds
Sulphur is the second member of oxygen family and belongs to group-16 (VI A) of the periodic table.
Occurrence : Sulphur occurs in the earth’s crust to the extent of 0.05%. It occurs in the free state as well as in combined state. Sulphur occurs mainly as sulphides and sulphates. eg.
| Sulphide Ores | Sulphate Ores |
| Iron pyrites (fool’s gold) – \[Fe{{S}_{2}}\] | Gypsum – \[CaS{{O}_{4}}.2{{H}_{2}}O\] |
| Galena – \[PbS\] | Epsom salt – \[MgS{{O}_{4}}.7{{H}_{2}}O\] |
| Copper pyrites – \[CuFe{{S}_{2}}\] | Barytes – \[BaS{{O}_{4}}\] |
| Cinnabar – \[HgS\] | Zinc blende – \[ZnS\] |
Extraction of sulphur (Frasch process) : Sulphur is generally extracted from underground deposits by drilling three concentric pipes upto the beds of sulphur (700 – 1200 feet deep).
Allotropy in sulphur : Sulphur exists in four allotropic forms,
(1) Rhombic or octahedral or a-sulphur : It is a bright yellow solid, soluble in \[C{{S}_{2}}\] and stable at room temp. All other varieties of sulphur gradually change into this form on standing.
(2) Monoclinic sulphur or prismatic or b-sulphur: It is prepared by melting the sulphur and then cooling it till a crust is formed.
On removing the crust, needle shaped crystals of monoclinic sulphur separate out. It is dull yellow in colour, soluble in \[C{{S}_{2}}\] and stable only above 369K. Below this temperature it changes into rhombic form.
Thus, at 369K both these varities co-exist. This temperature is called transition temperature and the two sulphurs are called enantiotropic substances. It also exist as molecules similar to that of rhombic sulphur but the symmetry of the crystals is different.
(3) Plastic or amorphous or \[\gamma \] -sulphur : It is a super cooled liquid insoluble in \[C{{S}_{2}}\], soft and amorphous. It consists of long zig-zag chains of S-atoms.
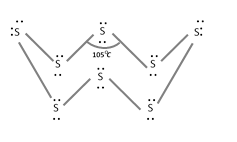
(4) Colloidal or \[\delta \]-sulphur : It is prepared by passing \[{{H}_{2}}S\] through a solution of an oxidizing agent or water or by treating sodium thiosulphate with dil. HCl.
Properties of sulphur : It burns in air with, a blue flame forming \[S{{O}_{2}}\], gives sulphur hexafluoride with \[{{F}_{2}}\] and sulphur mono chloride with\[C{{l}_{2}}\], sulphides with metals like Na, Ca, Zn, Hg, Fe, Cu etc., reduces \[HN{{O}_{3}}\] to \[N{{O}_{2}}\] and \[{{H}_{2}}S{{O}_{4}}\] to \[S{{O}_{2}}.\]With NaOH solution on heating,
\[{{S}_{8}}+12NaOH\xrightarrow{{}}4N{{a}_{2}}S+2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+6{{H}_{2}}O\].
It gives sodium sulphide and sodium thiosulphate, with excess of sulphur, \[2N{{a}_{2}}S+{{S}_{8}}\xrightarrow{{}}2N{{a}_{2}}{{S}_{5}}\].
Uses of sulphur : It is used in the manufacture of matches, gun powder (mixture of charcoal, sulphur and potassium nitrate), explosives and fire works \[S{{O}_{2}},{{H}_{2}}S{{O}_{4}}\], \[C{{S}_{2}}\] and dyes, sulpha drugs and ointment for curing skin diseases and in the vulcanization of rubber.
Compounds of Sulphur
(1) Hydrogen Sulphide : It is prepared in the laboratory by the action of dil. \[{{H}_{2}}S{{O}_{4}}\] on ferrous sulphide in kipp’s apparatus, \[FeS+{{H}_{2}}S{{O}_{4}}\to FeS{{O}_{4}}+{{H}_{2}}S\]. It is colourless gas having foul smell resembling that of rotten eggs. It reacts with many cations (of group II and IV) to give coloured sulphides,
\[C{{u}^{+2}}+{{S}^{-2}}\to \underset{\text{Black}}{\mathop{CuS}}\,\]; \[C{{d}^{+2}}+{{S}^{-2}}\to \underset{(\text{Yellow})}{\mathop{CdS}}\,\];
\[N{{i}^{+2}}+{{S}^{-2}}\to \underset{(\text{Black})}{\mathop{NiS}}\,\]; \[C{{o}^{+2}}+{{S}^{-2}}\to \underset{\text{(Black)}}{\mathop{CoS}}\,\]
The solubility of sulphides can be controlled by the \[{{H}^{+}}\]ions concentration and therefore, \[{{H}_{2}}S\] finds extensive use in qualitative analysis of cation radicals.

(2) Halides of sulphur : Two important halides of sulphur are \[S{{F}_{4}}\] and \[S{{F}_{6}}\].
(i) Sulphur tetrafluoride : \[S{{F}_{4}}\] is formed by the reaction of sulphur with \[Co{{F}_{3}}\].
\[S+4Co{{F}_{3}}\xrightarrow{{}}S{{F}_{4}}+4Co{{F}_{2}}\]
It is a colour gas which is quite reactive. It is hydrolysed with water.
\[S{{F}_{4}}+2{{H}_{2}}O\xrightarrow{{}}S{{O}_{2}}+4HF\]
It is used for fluorinating inorganic and organic compounds.
Structure : It has see-saw structure with \[s{{p}^{3}}d\]-hybrdization and is derived from triogonal bipyramid geometry in which an equatorial position is occupied by a lone pair of electrons.
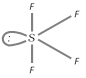
(ii) Sulphur hexafluoride : \[S{{F}_{6}}\] is prepared by burning sulphur in a stream of fluorine. OF6 is not known though sulphur forms SF6. This is because oxygen has no \[d\]-orbitals in its valence shell.
\[S{{F}_{6}}\] is a colourless gas. It is extremely inert substance even at red heat. It does not react with water. on account of its chemical inertness and dielectric strength, it is used as an insulator in high voltage generators and switch-gears.
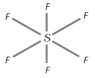
Structure : It has an octahedral structure with \[s{{p}^{3}}{{d}^{2}}\]-hybridisation around the central sulphur atom.
Therefore, all \[S-F\] bond distances are equal in its structure.
(3) Oxides of sulphur : Sulphur forms several oxides of which sulphur dioxide \[(S{{O}_{2}})\] and sulphur trioxide \[(S{{O}_{3}})\] are most important.
(i) Sulphur dioxide (SO2) : It is prepared by burning sulphur or iron pyrites in air.
\[{{S}_{8}}+8{{O}_{2}}\to 8S{{O}_{2}}\]; \[4Fe{{S}_{2}}+11{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}+8S{{O}_{2}}\]
In laboratory, it is prepared by heating copper turnings with conc. \[{{H}_{2}}S{{O}_{4}}\]
\[Cu+2{{H}_{2}}S{{O}_{4}}\to CuS{{O}_{4}}+S{{O}_{2}}+2{{H}_{2}}O\]
It is a colourless gas with irritating and suffocating smell.
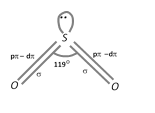
\[S{{O}_{2}}\] molecule has a bent structure with a O – S – O bond angle of 119o. Sulphur is \[s{{p}^{2}}\] hybridized.
(ii) Sulphur trioxide (SO3): It is formed by the oxidation of \[S{{O}_{2}}.\]
\[2S{{O}_{2}}+{{O}_{2}}\underset{{{V}_{2}}{{O}_{5}}}{\mathop{\xrightarrow{700K,\,2atm.}}}\,2S{{O}_{3}}\]
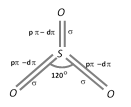
In the gaseous phase, it exists as planar triangular molecular species involving hybridization of the S-atom. It has three S–O \[\sigma \] bonds and three S–O \[\pi \]bonds. The O–S–O bond angle is of \[{{120}^{o}}\].
(4) Oxyacids of sulphur : Sulphur forms many oxyacids. Some of these are,
Oxyacids of sulphur
| Formula | Name | Important properties | Structural formula |
| \[{{H}_{2}}S{{O}_{3}}\](+4) | Sulphurous acid | Free acid does not exist diprotic, strong reducing agent | \[O=\underset{OH\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{.\,\,.\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{S-OH}}}\,}}\,\] |
| \[{{H}_{2}}S{{O}_{4}}\](+6) (Oil of vitriol) | Sulphuric acid | Stable diprotic, dehydrating agent | \[O\,\,=\underset{\,OH}{\overset{O\,\,\,}{\mathop{\underset{|\,\,\,}{\overset{||\,\,\,\,}{\mathop{S-}}}\,}}}\,OH\] |
| \[{{H}_{2}}{{S}_{2}}{{O}_{3}}\](?2 and +6) | Thiosulphuric acid | Free acid does not exist but its salts e.g. \[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\] All quite stable reducing agent | \[O=\underset{OH\,\,\,\,\,\,\,}{\overset{S\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\overset{||\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{S-OH}}}\,}}}\,\] |
| \[{{H}_{2}}{{S}_{2}}{{O}_{4}}\](+3) | Dithionous acid | \[HO-\overset{O}{\mathop{\overset{||}{\mathop{S}}\,}}\,-\overset{O}{\mathop{\overset{||}{\mathop{S}}\,}}\,-OH\] | |
| \[{{H}_{2}}{{S}_{2}}{{O}_{6}}\](+5) | Dithionic acid | Free acid is moderately stable but its salts are quite stable. | \[O=\underset{\,OH}{\overset{O\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\overset{||\,\,\,\,\,\,\,\,\,}{\mathop{S}}}\,}}}\,\underset{\,OH}{\overset{O\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\overset{||\,\,\,\,\,}{\mathop{S=}}}\,}}}\,O\] |
| \[{{H}_{2}}{{S}_{2}}{{O}_{7}}\](+6) (Oleum) | Disulphuric acid (Pyrosulphuric acid) | Strong oxidising agent | \[O=\underset{\,OH\,\,\,}{\overset{O\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,}{\overset{||\,\,\,\,\,\,\,\,\,}{\mathop{S-O}}}\,}}}\,-\underset{\,OH}{\overset{O\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\overset{||\,\,\,\,\,}{\mathop{S=}}}\,}}}\,O\] |
| \[{{H}_{2}}S{{O}_{5}}\](+6) (Caro's acid) | Peroxomonosulphuric acid (Its salts known as persulphates) | Stable crystalline solid, powerfull oxidising agent | \[HO-\underset{O\,\,\,\,}{\overset{O\,\,\,\,\,\,}{\mathop{\underset{||\,\,\,\,\,\,\,\,}{\overset{||\,\,\,\,\,\,\,\,\,}{\mathop{S}}}\,}}}\,OOH\] |
| \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\](+6) (Marshals acid) | Peroxodisulphuric acid (its salts are known as disulphates) | Strong oxidising agent. | \[O=\underset{\,OH}{\overset{O\,\,\,}{\mathop{\underset{|\,\,\,\,}{\overset{||\,\,\,\,\,}{\mathop{S-\,}}}\,}}}\,O-O-\underset{\,OH}{\overset{O\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,}{\overset{||\,\,\,\,\,}{\mathop{S=}}}\,}}}\,O\] |
Sulphuric acid \[({{H}_{2}}S{{O}_{4}})\]: \[{{H}_{2}}S{{O}_{4}}\] is a very stable oxyacid of sulphur.
It is often called king of chemicals, since it is one of the most useful chemicals in industry.
Manufacture of sulphuric acid : \[{{H}_{2}}S{{O}_{4}}\]can be manufactured by following process,
Lead chamber process : In this process, \[S{{O}_{2}}\] is oxidized to \[S{{O}_{3}}\] by the oxides of nitrogen and the \[S{{O}_{3}}\] thus formed is dissolved in steam to form \[{{H}_{2}}S{{O}_{4}}\].
\[S{{O}_{2}}+N{{O}_{2}}\] \[\to \]\[S{{O}_{3}}+NO\,\,;\]\[2NO+{{O}_{2}}\] \[\to \]\[2N{{O}_{2}}\]
\[S{{O}_{3}}+{{H}_{2}}O\] \[\to \]\[{{H}_{2}}S{{O}_{4}}\] .
Contact process : In the contact process, \[S{{O}_{2}}\] obtained by burning of S or iron pyrities is catalytically oxidized to \[S{{O}_{3}}\] in presence of finely divided Pt or \[{{V}_{2}}{{O}_{5}}\] as catalyst.
\[S+{{O}_{2}}\to S{{O}_{2}}\] or \[4Fe{{S}_{2}}+11{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}+8S{{O}_{2}}\]
\[2S{{O}_{2}}+{{O}_{2}}2S{{O}_{3}}.\]
\[{{V}_{2}}{{O}_{5}}\] is, however, preferred since is much cheaper than Pt and is also not poisoned by arsenic impurities.
The favorable conditions for maximum yield of \[S{{O}_{3}}\] are,
(a) High concentration of \[S{{O}_{2}}\] and \[{{O}_{2}}\]. (b) Low temperature of 673 to 723 K, (c) High pressure about 2 atmospheres.
\[S{{O}_{3}}\] thus obtained is absorbed in 98% \[{{H}_{2}}S{{O}_{4}}\] to form oleum which on dilution with water gives \[{{H}_{2}}S{{O}_{4}}\]of desired concentration.
\[S{{O}_{3}}+{{H}_{2}}S{{O}_{4}}\to \]\[\underset{\text{oleum}}{\mathop{{{H}_{2}}{{S}_{2}}{{O}_{7}}}}\,\]; \[{{H}_{2}}{{S}_{2}}{{O}_{7}}+{{H}_{2}}O\to 2{{H}_{2}}S{{O}_{4}}\]
Contact process is preferred over lead chamber process (gives 98% pure \[{{H}_{2}}S{{O}_{4}}\]) since it gives \[{{H}_{2}}S{{O}_{4}}\]of greater purity (100%).
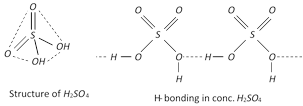
Structure : \[{{H}_{2}}S{{O}_{4}}\] is a covalent molecule with sulphur in a +6 oxidation state. The two oxygen atoms are linked to sulphur by double bonds while the other two oxygen atoms.
Are linked by single covalent bonds. Thus it has tetrahedral structure. Infact, sulphuric acid has an associated structure due to the presence of hydrogen bonds. As a result, it is a dense and viscous liquid and has a high boiling point of \[590K\]
Properties : \[{{H}_{2}}S{{O}_{4}}\] has high b.p. (611K) and is also highly viscous due to H-bonding. It has strong affinity for \[{{H}_{2}}O\] and a large amount of heat is evolved when it is mixed with water.
(i) \[{{H}_{2}}S{{O}_{4}}\] is a strong dibasic acid. It neutralizes alkalies, liberates \[C{{O}_{2}}\] from carbonates and bicarbonates.
(ii) It reacts with more electropositive (than hydrogen) metals to evolve \[{{H}_{2}}\] and produces \[S{{O}_{2}}\] on heating with less electropositive metals than hydrogen .eg.,
\[{{H}_{2}}S{{O}_{4}}+2KOH\to {{K}_{2}}S{{O}_{4}}+2{{H}_{2}}O\] ;
\[Cu+2{{H}_{2}}S{{O}_{4}}\to CuS{{O}_{4}}+S{{O}_{2}}+2{{H}_{2}}O\]
(iii) It is a strong oxidizing agent and oxidises as follows,
\[{{H}_{2}}S{{O}_{4}}\to {{H}_{2}}O+S{{O}_{2}}+O\]
\[C+2{{H}_{2}}S{{O}_{4}}\to 2SO+CO+2{{H}_{2}}O\]
\[S+2{{H}_{2}}S{{O}_{4}}\to 3S{{O}_{2}}+2{{H}_{2}}O\]
\[{{P}_{4}}+10{{H}_{2}}S{{O}_{4}}\to 4{{H}_{2}}P{{O}_{4}}+10S{{O}_{2}}+4{{H}_{2}}O\]
\[2HBr+{{H}_{2}}S{{O}_{4}}\to B{{r}_{2}}+2{{H}_{2}}O+S{{O}_{2}}\]
\[2HI+{{H}_{2}}S{{O}_{4}}\to 2{{H}_{2}}O+{{I}_{2}}+2S{{O}_{2}}\]
(iv) It reacts with number of salts. It liberates HCl from chlorides, \[{{H}_{2}}S\]from sulphides, \[HN{{O}_{3}}\] from nitrates.
(v) It acts as a strong dehydrating agent, as it dehydrates, sugar to sugar charcoal (carbon), formic acid to CO, oxalic acid to \[CO+C{{O}_{2}}\] and ethyl alcohol to ethylene.
(vi) It is also a good sulphonating agent and used for sulphonation of aromatic compounds. eg.,
Flow sheet diagram of it?s preparation is as follows
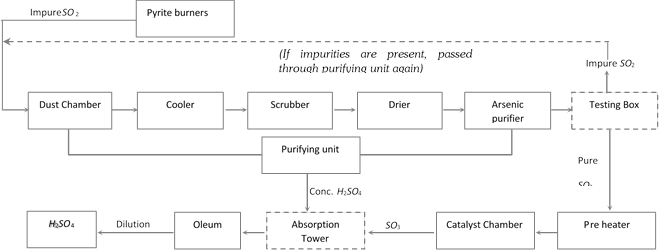
\[BaC{{l}_{2}}+{{H}_{2}}S{{O}_{4}}\to \underset{\text{(white ppt)}}{\mathop{BaS{{O}_{4}}}}\,+\underset{{}}{\mathop{2HCl}}\,\]
\[Pb{{(N{{O}_{3}})}_{2}}+{{H}_{2}}S{{O}_{4}}\to \underset{\text{(white ppt}\text{.)}}{\mathop{PbS{{O}_{4}}}}\,+\underset{{}}{\mathop{2HN{{O}_{3}}}}\,\]
\[\underset{\text{Sugar}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,\xrightarrow{\text{Conc}\text{. }{{\text{H}}_{\text{2}}}S{{O}_{4}}}\underset{\text{Carbon}}{\mathop{12C}}\,+11{{H}_{2}}O\]
\[HCOOH\xrightarrow{Conc.{{H}_{2}}S{{O}_{4}}}CO+{{H}_{2}}O\]
Uses : \[{{H}_{2}}S{{O}_{4}}\] is used (i) in the preparation of fertilizers like \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\] and super phosphate of lime, (ii) in lead storage batteries (iii) in preparation of dyes, paints and explosives (iv) in textile and paper industry (v) for training of tanning (vi) as a dehydrating agent.
(5) Sodium thiosulphate \[N{{a}_{2}}{{S}_{2}}{{O}_{3}}.5{{H}_{2}}O\]: It is manufactured by saturating a solution of sodium carbonate with \[S{{O}_{2}}\] which gives a solution of sodium sulphite,
\[N{{a}_{2}}C{{O}_{3}}+S{{O}_{2}}+{{H}_{2}}O\to N{{a}_{2}}S{{O}_{3}}+C{{O}_{2}}+{{H}_{2}}O\]
The resulting solution is boiled with powdered sulphur as, \[N{{a}_{2}}S{{O}_{3}}+S\]\[\xrightarrow{373K}\]\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}\]
The solution is then cooled to get crystals of sodium thiosulphate.
Physical properties : (i) Sodium thiosulphate is a colourless crystalline solid. In the hydrated form, it is called hypo. (ii) It melts at 320 K and loses its water molecules of crystallization on heating to 490K.
Chemical properties
(i) Action with halogens : It reacts with halogens as,
(a) Chlorine water oxidizes sodium thiosulphate to sodium sulphate and sulphur is precipitated,
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}+C{{l}_{2}}+{{H}_{2}}O\to 2HCl+N{{a}_{2}}S{{O}_{4}}S\]
This property enables it to act as an antichlor in bleaching i.e. it destroys the unreacted chlorine in the process of bleaching.
(b) Bromine water also oxidizes sodium thiosulphate to sodium sulphate and sulphur,
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}+B{{r}_{2}}+{{H}_{2}}O\to ~N{{a}_{2}}S{{O}_{4}}+2HBr+S\]
(c) With iodine it forms a soluble compound called sodium tetrathionate,
\[2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{{I}_{2}}\to \underset{\text{Sod}\text{. tetrathionate}}{\mathop{N{{a}_{2}}{{S}_{4}}{{O}_{6}}}}\,+2NaI\]
Therefore, hypo is commonly used to remove iodine stains from the clothes.
(ii) Action of heat : Upon heating, sodium thiosulphate decomposes to form sodium sulphate and sodium pentasulphide, \[4N{{a}_{2}}{{S}_{2}}{{O}_{3}}\xrightarrow{\text{Heat}}3N{{a}_{2}}S{{O}_{4}}+\underset{\text{Sodium pentasulphide}}{\mathop{N{{a}_{2}}{{S}_{5}}}}\,\]
(iii) Action with acids : Sodium thiosulphate reacts with dilute hydrochloric acid or Sulphuric acid forming sulphur dioxide and sulphur. The solution turns milky yellow due to sulphur.
\[N{{a}_{2}}{{S}_{2}}{{O}_{3}}+\text{ }2HCl\to ~2NaCl+S{{O}_{2}}+{{H}_{2}}O+S\]
(iv) Action with silver halides : Sodium thiosulphate forms soluble complex when treated with silver chloride or silver bromide, \[2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+2AgBr\to \underset{\begin{smallmatrix}
\text{Sodium dithiosulphate } \\
\text{argentate (I) compex}
\end{smallmatrix}}{\mathop{N{{a}_{3}}Ag{{({{S}_{2}}{{O}_{3}})}_{2}}}}\,+NaBr\].
This property of hypo is made use in photography.
Uses of sodium thiosulphate
(i) It is largely used in photography as a fixing agent.
(ii) It is used as a preservative for fruit products such as jams and squashes.
(iii) It is used as an antichlor in bleaching.
(iv) It is used as a volumetric agent for the estimation of iodine.
(v) It is used in medicine.
You need to login to perform this action.
You will be redirected in
3 sec
