Iron And Its Compounds
Category : JEE Main & Advanced
(1) Ores of iron : Haematite \[F{{e}_{2}}{{O}_{3}}\], Magnetite \[(F{{e}_{3}}{{O}_{4}}),\] Limonite \[(F{{e}_{2}}{{O}_{3}}.3{{H}_{2}}O)\], Iron pyrites \[(Fe{{S}_{2}}),\] Copper pyrities \[(CuFe{{S}_{2}})\] etc.
(2) Extraction : Cast iron is extracted from its oxides by reduction with carbon and carbon monoxide in a blast furnace to give pig iron.
Roasting : Ferrous oxide convert into ferric oxide.
\[F{{e}_{2}}{{O}_{3}}.\,3{{H}_{2}}O\to F{{e}_{2}}{{O}_{3}}+3{{H}_{2}}O\];\[2FeC{{O}_{3}}\to 2FeO+2C{{O}_{2}}\]
\[4FeO+{{O}_{2}}\to 2F{{e}_{2}}{{O}_{3}}\]
Smelting : Reduction of roasted ore of ferric oxide carried out in a blast furnace.
(i) The reduction of ferric oxide is done by carbon and carbon monoxide (between 1473k to 1873k)
\[2C+{{O}_{2}}\to 2CO\]
(ii) \[F{{e}_{2}}{{O}_{3}}+3CO2Fe+3C{{O}_{2}}\]. It is a reversible and exothermic reaction. Hence according to Le-chatelier principle more iron will be produced in the furnace at lower temp. \[\underset{\text{(it is not reversible)}}{\mathop{F{{e}_{2}}{{O}_{3}}+CO\to 2FeO+C{{O}_{2}}}}\,\]
(iii) \[FeO+C\underset{\begin{smallmatrix}
\text{endothermic } \\
\text{ reaction}
\end{smallmatrix}}{\mathop{\xrightarrow{1073\,K}}}\,\] \[Fe+CO\]
The gases leaving at the top of the furnace contain up to 28% CO and are burnt in cowper's stove to pre-heat the air for blast
Varieties of iron : The three commercial varieties of iron differ in their carbon contents. These are;
(1) Cast iron or Pig-iron : It is most impure form of iron and contains highest proportion of carbon (2.5–4%).
(2) Wrought iron or Malleable iron : It is the purest form of iron and contains minimum amount of carbon (0.12–0.25%).
(3) Steel : It is the most important form of iron and finds extensive applications. Its carbons content (Impurity) is mid-way between cast iron and wrought iron. It contains 0.2–1.5% carbon. Steels containing 0.2–0.5% of carbon are known as mild steels, while those containing 0.5–1.5% carbon are known as hard steels.
Steel is generally manufactured from cast iron by three processes, viz, (i) Bessemer Process which involves the use of a large pear-shaped furnace (vessel) called Bessemer converter, (ii) L.D. process and (iii) open hearth process, Spiegeleisen (an alloy of Fe, Mn and C) is added during manufacture of steel.
Heat treatment of steels : Heat treatment of steel may be defined as the process of carefully heating the steel to high temperature followed by cooling to the room temperature under controlled conditions. Heat treatment of steel is done for the following two purposes,
(i) To develop certain special properties like hardness, strength, ductility etc. without changing the chemical composition.
(ii) To remove some undesirable properties or gases like entrapped gases, internal stresses and strains. The various methods of heat treatment are,
(a) Annealing : It is a process of heating steel to redness followed by slow cooling.
(b) Quenching or hardening : It is a process of heating steel to redness followed by sudden cooling by plunging the red hot steel into water or oil.
(c) Tempering : It is a process of heating the hardened or quenched steel to a temperature much below redness (473–623K) followed by slow cooling.
(d) Case-hardening : It is a process of giving a thin coating of hardened steel to wrought iron or to a strong and flexible mild steel by heating it in contact with charcoal followed by quenching in oil.
(e) Nitriding : It is a process of heating steels at about \[700{{\,}^{o}}C\] in an atmosphere of ammonia. This process imparts a hard coating of iron nitride on the surface of steel.
Properties of steel : The properties of steel depend upon its carbon contents. With the increase in carbon content, the hardness of steel increases while its ductility decreases.
(i) Low carbon or soft steels contain carbon upto 0.25%.
(ii) Medium carbon steels or mild steels contain 0.25–0.5% carbon.
(iii) High carbon or hard steels contains 0.1 – 1.5 percent carbon.
(iv) Alloy steels or special steels are alloys of steel with \[Ni,\,Cr,\,Co,\,W,\,Mn,\,V\] etc., For example
(a) Stainless steel (Fe = 73%, Cr = 18%, Ni = 8% + C) is resistant to corrosion and is used for making ornamental pieces, cutlery etc.
(b) Invar (Fe = 64%, Ni = 36%) has small coefficient of expansion and is used for making metre scales, pendulum rods and watches.
(c) Manganese steel (Fe = 86%, Mn 13% + carbon) is very hard and resistant to wear and hence is used for making rock drills, safes etc.
(d) Tungsten steel (Fe = 94%, W = 5% + carbon) is quite hard and is used for making high speed cutting tools.
(e) Permalloy (Fe = 21%, Ni = 78% + carbon) is strongly magnetised by electric current but loses magnetism when current is cut off. It is used for making electromagnets, ocean cables etc.
Properties of iron
(1) Dry or moist air has no action on pure iron but impure iron when exposed to moist air is covered with a layer of rust \[F{{e}_{2}}{{O}_{3}}+Fe{{(OH)}_{3}}\]. However, finely divided pure iron burns in air or oxygen forming \[F{{e}_{3}}{{O}_{4}}\] (magnetic oxide of iron).
\[3Fe+2{{O}_{2}}\to F{{e}_{3}}{{O}_{4}}\]
(2) Iron decomposes steam at red heat
\[3Fe+\underset{\text{Steam}}{\mathop{4{{H}_{2}}O}}\,\xrightarrow{\text{Red heat}}F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}\]
(3) Action of acids : Iron reacts with dil. HCl and dil.\[{{H}_{2}}S{{O}_{4}}\] liberating hydrogen. with hot conc.\[{{H}_{2}}S{{O}_{4}}\], it gives \[S{{O}_{2}}\], with dil.\[HN{{O}_{3}}\], it gives \[N{{H}_{4}}N{{O}_{3}}\] and moderately conc.\[HN{{O}_{3}}\] reacts with iron forming \[N{{O}_{2}}\].
Cold conc. \[HN{{O}_{3}}\] makes iron passive due to the deposit of a thin layer of iron oxide \[(F{{e}_{3}}{{O}_{4}})\] on the surface.
Hot conc.\[HN{{O}_{3}}\] reacts with iron liberating NO.
\[Fe+4HN{{O}_{3}}(\text{hot conc}\text{.})\to Fe{{(N{{O}_{3}})}_{3}}+NO+2{{H}_{2}}O\]
(4) Iron does not react with alkalies.
(5) It displaces less electropositive metals (e.g., Cu, Ag etc.) from their salts
\[CuS{{O}_{4}}+Fe\to FeS{{O}_{4}}+Cu\]
(6) Finely divided iron combines with CO forming penta carbonyl
\[Fe+5CO\to Fe{{(CO)}_{5}}\]
(7) Iron does not form amalgam with Hg.
(8) Iron is the most abundant and most widely used transition metal.
Compounds of iron
(1) Oxides of Iron : Iron forms three oxides \[FeO,\,F{{e}_{2}}{{O}_{3}}\] (Haematite), \[F{{e}_{3}}{{O}_{4}}\](magnetite also called magnetic oxide or load stone).
(i) Ferrous oxide, \[FeO:\] It is a black powder, basic in nature and reacts with dilute acids to give ferrous salts.
\[FeO+{{H}_{2}}S{{O}_{4}}\to FeS{{O}_{4}}+{{H}_{2}}O\]; It is used in glass industry to impart green colour to glass.
(ii) Ferric oxide \[F{{e}_{2}}{{O}_{3}}:\] It is a reddish brown powder, not affected by air or water; amphoteric in nature and reacts both with acids and alkalis giving salts. It can be reduced to iron by heating with C or CO.
\[F{{e}_{2}}{{O}_{3}}+3C\to 2Fe+3CO\]; \[F{{e}_{2}}{{O}_{3}}+3CO\to 2Fe+3C{{O}_{2}}\]
It is used as red pigment to impart red colour to external walls and as a polishing powder by jewellers.
(iii) Ferrosoferricoxide \[F{{e}_{3}}{{O}_{4}}(FeO.\,F{{e}_{2}}{{O}_{3}}):\] It is more stable than \[FeO\] and \[F{{e}_{2}}{{O}_{3}},\] magnetic in nature and dissolves in acids giving a mixture of iron (II) and iron (III) salts.
\[F{{e}_{3}}{{O}_{4}}+4{{H}_{2}}S{{O}_{4}}\](dil) \[\to FeS{{O}_{4}}+F{{e}_{2}}{{(S{{O}_{4}})}_{3}}+4{{H}_{2}}O\]
(2) Ferrous sulphide \[FeS\,:\] It is prepared by heating iron filing with sulphur. With dilute \[{{H}_{2}}S{{O}_{4}},\] it gives \[{{H}_{2}}S.\] \[FeS+{{H}_{2}}S{{O}_{4}}\](dil) \[\to FeS{{O}_{4}}+{{H}_{2}}S\uparrow \]
(3) Ferric chloride \[FeC{{l}_{3}}:\] (i) preparation : It is prepared by treating \[Fe{{(OH)}_{3}}\]with \[HCl\]
\[Fe{{(OH)}_{3}}+3HCl\to FeC{{l}_{3}}+3{{H}_{2}}O\]
The solution on evaporation give yellow crystals of \[FeC{{l}_{3}}.\,6{{H}_{2}}O\]
(ii) Properties : (a) Anhydrous \[FeC{{l}_{3}}\] forms reddish-black deliquescent crystals.
(b) \[FeC{{l}_{3}}\] is hygroscopic and dissolves in \[{{H}_{2}}O\] giving brown acidic solution due to formation of \[HCl\]
\[FeC{{l}_{3}}+3{{H}_{2}}O\to \underset{\text{(Brown) }}{\mathop{Fe{{(OH)}_{3}}}}\,+3HCl\]
(c) Due to oxidising nature \[F{{e}^{3+}}\] ions \[FeC{{l}_{3}}\] is used in etching metals such as copper
\[2F{{e}^{3+}}+Cu\to 2F{{e}^{2+}}+C{{u}^{2+}}(aq)\]
(d) In vapour state \[FeC{{l}_{3}}\] exists as a dimer, \[F{{e}_{2}}C{{l}_{6}}\]
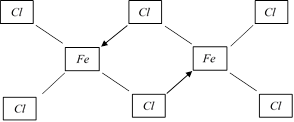
(e) \[FeC{{l}_{3}}\] is used as stypic to stop bleeding from a cut.
(4) Ferrous sulphate, \[FeS{{O}_{4}}\],\[7{{H}_{2}}O\] (Green vitriol) : It is prepared as follow ,
\[Fe+{{H}_{2}}S{{O}_{4}}\to FeS{{O}_{4}}+{{H}_{2}}\]
(i) One pressure to moist air crystals become brownish due to oxidation by air.
\[4FeS{{O}_{4}}+2{{H}_{2}}O+{{O}_{2}}\to 4Fe(OH)S{{O}_{4}}\]
(ii) On heating, crystals become anhydrous and on strong heating it decomposes to \[F{{e}_{2}}{{O}_{3}},\,S{{O}_{2}}\] and \[S{{O}_{3}}\].
\[FeS{{O}_{4}}.7{{H}_{2}}O\xrightarrow{\text{heat}}FeS{{O}_{4}}+7{{H}_{2}}O\] \[2FeS{{O}_{4}}\underset{\text{heating }}{\mathop{\xrightarrow{Strong}}}\,F{{e}_{2}}{{O}_{3}}+S{{O}_{2}}+S{{O}_{3}}\]
(iii) It can reduce acidic solution of \[KMn{{O}_{4}}\] and \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
(iv) It is generally used in double salt with ammonium sulphate.
\[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}+FeS{{O}_{4}}+6{{H}_{2}}O\to \underset{\text{Mohr }\!\!'\!\!\text{ s salt}}{\mathop{FeS{{O}_{4}}.{{(N{{H}_{4}})}_{2}}}}\,\,S{{O}_{4}}.6{{H}_{2}}O\]
Mohr’s salt is resistant to atmospheric oxidation.
(v) It is used in the ring test for nitrate ions where it gives brown coloured ring of compound \[FeS{{O}_{4}}.\,NO.\]
\[FeS{{O}_{4}}+NO\to FeS{{O}_{4}}.NO\]
(vi) \[FeS{{O}_{4}}\] is used in manufacture of blue black ink.
(vii) \[FeS{{O}_{4}}+{{H}_{2}}{{O}_{2}}\] is known as a name of Fenton’s reagent.
(5) Mohr's salt \[FeS{{O}_{4}}\,.\,{{(N{{H}_{4}})}_{2}}\,S{{O}_{4}}.\,6{{H}_{2}}O:\] It is a double salt and is prepared by crystallising a solution containing equivalent amounts of \[FeS{{O}_{4}}.7{{H}_{2}}O\] and \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\]. It may be noted that Mohr’s salt contains only \[F{{e}^{2+}}\] ions without any trace of \[F{{e}^{3+}}\] ions. In contrast \[FeS{{O}_{4}}.7{{H}_{2}}O\] always contains some \[F{{e}^{3+}}\] ions due to aerial oxidation of \[F{{e}^{2+}}\] ions. Mohr salt is, therefore, used as a primary standard in volumetric analysis since a standard solution of \[F{{e}^{2+}}\] ions can be obtained directly by weighing a known amount of the Mohr salt.
It acts as a reducing agent and as such reduces acidified \[KMn{{O}_{4}}\] and \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] solutions.
\[MnO_{4}^{-}+5F{{e}^{2+}}+8{{H}^{+}}\to 5F{{e}^{3+}}+M{{n}^{2+}}+4{{H}_{2}}O\]
\[C{{r}_{2}}O_{7}^{2-}\underset{\text{(From mohrs salt)}}{\mathop{+6F{{e}^{2+}}+}}\,14{{H}^{+}}\to 6F{{e}^{3+}}+2C{{r}^{3+}}+7{{H}_{2}}O\]
You need to login to perform this action.
You will be redirected in
3 sec
