Electronic Configurations Of Elements
Category : JEE Main & Advanced
On the basis of the elecronic configuration principles the electronic configuration of various elements are given in the following table :
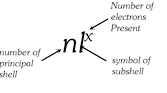
The above method of writing the electronic configurations is quite cumbersome. Hence, usually the electronic configuration of the atom of any element is simply represented by the notation.
Some Unexpected Electronic Configuration
Some of the exceptions are important though, because they occur with common elements, notably chromium and copper.
\[Cu\] has 29 electrons. Its excepted electronic configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{9}}\] but in reality the configuration is \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{10}}\] as this configuration is more stable. Similarly \[Cr\] has the configuration of \[1{{s}^{2}}2{{s}^{2}}s{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{1}}3{{d}^{5}}\] instead of \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{4}}\].
Factors responsible for the extra stability of half-filled and completely filled subshells,
(i) Symmetrical distribution : It is well known fact that symmetry leads to stability. Thus the electronic configuration in which all the orbitals of the same subshell are either completely filled or are exactly half filled are more stable because of symmetrical distribution of electrons.
(ii) Exchange energy : The electrons with parallel spins present in the degenerate orbitals tend to exchange their position. The energy released during this exchange is called exchange energy. The number of exchanges that can take place is maximum when the degenerate orbtials (orbitals of same subshell having equal energy) are exactly half-filled or completely. As a result, the exchange energy is maximum and so it the stability.
You need to login to perform this action.
You will be redirected in
3 sec
