Biodegradable Polymers
Category : JEE Main & Advanced
These are the polymers which are degraded by micro-organisms within a suitable period so that biodegradable polymers and their degraded products do not cause any serious affects on the environment.
In biological systems, biopolymers degrade mainly by enzymatic hydrolysis and to some extent by oxidation. Therefore, in view of the disposal problems of polymer waste and for developing polymers for other safe uses in human systems, attempts have been made to develop biodegradable synthetic polymers. These synthetic polymers mostly have functional groups which are normally present in biopolymers and lipids.
Among these aliphatic polyesters are one important class of biodegradable polymers which are commercially potential biomaterials. The common examples of biodegradable polymers are polyhydroxy butyrate (PHB), polyhydroxy butyrate –co-\[\beta \]-hydroxy valerate (PHBV), polyglycolic acid (PGA), polylactic acid (PLA), poly (Î-caprolactone) (PCL), etc.
Uses : Biodegradable polymers are used mainly for medical goods such as surgical sutures, tissue in growth materials or for controlled drug release devices, plasma substitutes etc. The decomposition reactions usually involve hydrolysis (either enzymatically induced or by non-enzymatic mechanisms) to non-toxic small molecules which can be metabolized by or excreted from the body. These are also finding use in agriculture materials (such as films, seed coatings), fast food wrappers, personal hygiene products, etc.
(i) Polyhydroxy butyrate (PHB)
Polyhydroxy butyrate (PHB) is obtained from hydroxy butyric acid (3-hydroxy butanoic acid)
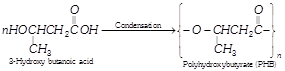
(ii) Poly-Hydroxybutyrate-co-\[\beta \]-Hydroxy valerate (PHBV) : It is copolymer of 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid, in which the monomer units are joined by ester linkages.
\[\underset{\text{3-Hydroxy butanoic acid}}{\mathop{nC{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}COOH}}\,\,+\,n\underset{\text{3-Hydroxy pentanoic acid}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-\underset{OH}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}-COOH}}\,\]\[\to \underset{\text{PHBV}}{\mathop{{{\left( -O-\underset{R}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{2}}-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,O- \right)}_{n}}}}\,\], \[R=C{{H}_{3}}\], \[{{C}_{2}}{{H}_{5}}\]
The properties of PHBV vary according to the ratio of both the acids. 3-Hydroxy butanoic acid provides stiffness while 3-Hydroxypentanoic acid gives flexibility to the copolymer.
(iii) Polyglycolic acid (PGA) : Polyglycolic acid (PGA) is obtained by the chain polymerisation of cyclic dimer of glycolic acid, \[HO-C{{H}_{2}}-COOH\].
\[\underset{\text{Glycolic acid}}{\mathop{nHO-C{{H}_{2}}COOH}}\,\xrightarrow{\text{Heat}}\underset{\text{Polyglycolic acid (PGA)}}{\mathop{{{\left( -OC{{H}_{2}}\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,- \right)}_{n}}}}\,\]
(iv) Polylactic acid (PLA) : Polylactic acid (PLA) is obtained by polymerisation of the cyclic dimer of lactic acid \[(HO-CH(C{{H}_{3}})COOH)\] or by microbiological synthesis of lactic acid followed by the polycondensation and removal of water by evaporation.
\[\underset{\text{Lactic acid}}{\mathop{nHO\underset{C{{H}_{3}}\,}{\mathop{\underset{|}{\mathop{C}}\,H\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,}}\,-OH}}\,\xrightarrow{\text{Condensati}\text{on}}\underset{\text{Polylactic acid (PLA)}}{\mathop{{{\left( -O\underset{C{{H}_{3}}\,}{\mathop{\underset{|}{\mathop{C}}\,H-}}\,\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,- \right)}_{n}}}}\,\]
(v) Poly (\[\in \]-caprolactone) (PCL) : It is obtained by chain polymerisation of the lactone of 6-hydroxy hexanoic acid.
\[\underset{\text{PCL}}{\mathop{{{\left( -O-{{(C{{H}_{2}})}_{2}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,- \right)}_{n}}}}\,\]
Uses : PGA and PLA (90 : 10) is used to make absorbable structure to close an internal of external wound and has replaced cat gut these are completely degraded and absorbed by the body within 15 days to one month of the surgery.
Polyhydroxybutyrate (PHB) and (PHBV) have been used for making films for packaging and into moulded items.
You need to login to perform this action.
You will be redirected in
3 sec
