Some More Important Halogen Derivatives
Category : JEE Main & Advanced
(1) Freons : The chloro fluoro derivatives of methane and ethane are called freons. Some of the derivatives are: \[CH{{F}_{2}}Cl\] (monochlorodifluoromethane), \[C{{F}_{2}}C{{l}_{2}}\] (dichlorodifluoro-methane), \[HC{{F}_{2}}CHC{{l}_{2}}\] (1,1-dichloro-2,2-difluoroethane). These derivatives are non-inflammable, colourless, non-toxic, low boiling liquids. These are stable upto 550°C. The most important and useful derivative is \[C{{F}_{2}}C{{l}_{2}}\] which is commonly known as freon and freon-12.
Freon or freon-12 \[(C{{F}_{2}}C{{l}_{2}})\] is prepared by treating carbon tetrachloride with antimony trifluoride in the presence of antimony pentachloride as a catalyst.
\[3CC{{l}_{4}}+2Sb{{F}_{3}}\underset{\text{Catalyst}}{\mathop{\xrightarrow{SbC{{l}_{5}}}}}\,3CC{{l}_{2}}{{F}_{2}}+2SbC{{l}_{3}}\]
Or it can be obtained by reacting carbon tetrachloride with hydrofluoric acid in presence of antimony pentafluoride.
\[CC{{l}_{4}}+2HF\xrightarrow{Sb{{F}_{5}}}CC{{l}_{2}}{{F}_{2}}+2HCl\]
Under ordinary conditions freon is a gas. Its boiling point is \[-{{29.8}^{o}}C\]. It can easily be liquified. It is chemically inert. It is used in air-conditioning and in domestic refrigerators for cooling purposes (As refrigerant). It causes depletion of ozone layer.
(2) Teflon : It is plastic like substance produced by the polymerisation of tetrafluoroethylene \[(C{{F}_{2}}=C{{F}_{2}})\].
Tetrafluoroethylene is formed when chloroform is treated with antimony trifluoride and hydrofluoric acid.
\[CHC{{l}_{3}}\underset{HF}{\mathop{\xrightarrow{Sb{{F}_{3}}}}}\,CH{{F}_{2}}Cl\underset{-HCl}{\mathop{\xrightarrow{800{}^\circ C}}}\,\underset{\text{(b}\text{.pt}\text{.-76}{}^\circ \text{C)}}{\mathop{C{{F}_{2}}=C{{F}_{2}}}}\,\]
On polymerisation tetrafluoroethylene forms a plastic-like material which is called teflon.
\[\underset{\text{Tetrafluoroethylene}}{\mathop{nC{{F}_{2}}=C{{F}_{2}}}}\,\xrightarrow{{}}\underset{\text{Teflon}}{\mathop{{{(-C{{F}_{2}}-C{{F}_{2}}-)}_{n}}}}\,\]
Teflon is chemically inert substance. It is not affected by strong acids and even by boiling aqua-regia. It is stable at high temperatures. It is, thus, used for electrical insulation, preparation of gasket materials and non-sticking frying pans.
(3) Acetylene tetrachloride (Westron), CHCl2?CHCl2 : Acetylene tetrachloride is also known as sym. tetrachloroethane. It is prepared by the action of chlorine on acetylene in presence of a catalyst such as ferric chloride, aluminium chloride, iron, quartz or kieselguhr.
\[CH\equiv CH+2C{{l}_{2}}\xrightarrow{{}}\underset{\text{(1,1,2,2}-\text{Tetrachloroethane)}}{\mathop{CHC{{l}_{2}}\cdot CHC{{l}_{2}}}}\,\]
In absence of catalyst, the reaction between chlorine and acetylene is highly explosive producing carbon and HCl. The reaction is less violent in presence of a catalyst.
It is a heavy, non-inflammable liquid. It boils at \[{{146}^{o}}C\]. It is highly toxic in nature. Its smell is similar to chloroform. It is insoluble in water but soluble in organic solvents.
On further chlorination, it forms penta and hexachloroethane. On heating with lime (Calcium hydroxide), it is converted to useful product westrosol \[(CC{{l}_{2}}=CHCl)\].
\[\underset{\text{Westron}}{\mathop{2CHC{{l}_{2}}-CHC{{l}_{2}}}}\,+Ca{{(OH)}_{2}}\xrightarrow{{}}\underset{\text{(Trichloroethene)}}{\mathop{\underset{\text{Westrosol}}{\mathop{2CHCl=CC{{l}_{2}}}}\,}}\,+CaC{{l}_{2}}+2{{H}_{2}}O\]\[\underset{\text{(Trichloroethene)}}{\mathop{\underset{\text{Westrosol}}{\mathop{2CHCl=CC{{l}_{2}}}}\,}}\,+CaC{{l}_{2}}+2{{H}_{2}}O\]
Both westron and westrosol are used as solvents for oils, fats, waxes, resins, varnishes and paints, etc.
(4) p-Dichlorobenzene : It is prepared by chlorination of benzene.
It is a white, volatile solid having melting point of 325 K, which readily sublimes. It resembles chlorobenzene in their properties.
It is used as general insecticides, germicide, soil fumigant deodorant. It is used as a larvicide for cloth moth and peach tee borer.
(5) DDT; 2, 2-bis (p-Chlorophenyl) –1,1,1-trichloroethane :
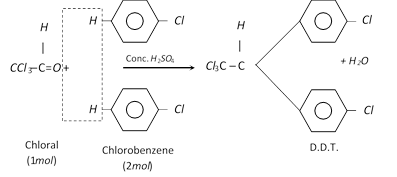
Properties and uses of D.D.T.
(i) D.D.T. is almost insoluble in water but it is moderately soluble in polar solvents.
(ii) D.D.T. is a powerful insecticide. It is widely used as an insecticide for killing mosquitoes and other insects.
Side Effects of D.D.T. : D.D.T. is not biodegradable. Its residues accumulate in environment and its long term effects could be highly dangerous. It has been proved to be toxic to living beings. Therefore, its use has been abandoned in many western countries. However, inspite of its dangerous side effects, D.D.T. is still being widely used in India due to non-availability of other cheaper insecticides.
(6) BHC (Benzene hexachloride), C6H6Cl6 :

Uses : It is an important agricultural pesticide mainly used for exterminating white ants, leaf hopper, termite, etc. It is also known by the common name gammaxene or lindane or 666.
(7) Perfluorocarbons (PFCs) : Perfluorocarbons \[({{C}_{n}}{{F}_{2n+2}})\] are obtained by controlled fluorination of vapourized alkanes diluted with nitrogen gas in the presence of a catalyst.
\[{{C}_{7}}{{H}_{16}}+16{{F}_{2}}\underset{Co{{F}_{2}}\text{(Catalyst)}}{\mathop{\xrightarrow{\text{Vapour phase, }{{N}_{2}},573K}}}\,\underset{\text{Perfluoroheptane}}{\mathop{{{C}_{7}}{{F}_{16}}}}\,+16HF\]
These are colourless, odourless, non-toxic, non-corrosive, non-flammable, non-polar, extremely stable and unreactive gases, liquids and solids. These are stable to ultraviolet radiations and other ionising radiations and therefore, they do not deplete the ozone layer like freons.
These are good electrical insulators. These have many important uses such as :
(i) These are used as lubricants, surface coatings and dielectrics.
(ii) These are used as heat transfer media in high voltage electrical equipment.
(iii) These are used for vapour phase soldering, gross leak detection of sealed microchips etc. in electronic industry.
(iv) These are also used in health care and medicine such as skin care cosmetics, wound healing, liquid ventilation, carbon monoxide poisoning and many medical diagnosis.
You need to login to perform this action.
You will be redirected in
3 sec
