Allyl iodide or 3-iodopropene-1, \[IC{{H}_{\mathbf{2}}}CH\text{ }=\text{ }C{{H}_{\mathbf{2}}}\]
Category : JEE Main & Advanced
(1) Synthesis : It is obtained,
(i) \[\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,+C{{l}_{2}}\xrightarrow{500{}^\circ C}\underset{Cl\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,\underset{\text{Allyl chloride}\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-CH=C{{H}_{2}}}}\,\]
Or \[\underset{\text{Allyl}\,\text{alcohol}}{\mathop{3\underset{OH\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-CH=C{{H}_{2}}}}\,+PC{{l}_{3}}\xrightarrow{\text{Heat}}3\underset{Cl\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-CH=C{{H}_{2}}+{{H}_{3}}P{{O}_{3}}\]
\[\underset{\text{Allyl}\,\text{chloride}}{\mathop{\underset{Cl\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}-}}\,CH=C{{H}_{2}}+NaI}}\,\underset{\text{Heat}}{\mathop{\xrightarrow{\text{Acetone}}}}\,\underset{\text{Allyl}\,\text{iodide}}{\mathop{\underset{I\,\,\,\,\,\,\,}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}}}\,-CH=C{{H}_{2}}}}\,+NaCl\]
This is halogen- exchange reaction and is called Finkelstein reaction.
(ii) \[\underset{\text{Glycerol}}{\mathop{\begin{matrix} \underset{\,\,\,|}{\mathop{\,\,C}}\,{{H}_{2}}OH \\ \underset{|}{\mathop{C}}\,HOH \\ \,\,C{{H}_{2}}OH \\ \end{matrix}}}\,+3HI\xrightarrow[-3{{H}_{2}}O]{}\underset{\text{1,2,3-Tri-iodopropane}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\,\,\,\,\,\,\begin{matrix} \underset{|}{\mathop{C}}\,{{H}_{2}}I \\ \underset{|}{\mathop{C}}\,HI\,\,\, \\ C{{H}_{2}}I \\ \end{matrix}\,\,\,\,\,\,\,\underset{-{{I}_{2}}}{\mathop{\xrightarrow{\text{Heat}}}}\,\,\,\,\,\,\,\,}}\,\underset{\text{Allyl}\,\text{iodide}}{\mathop{\begin{matrix} \underset{|}{\mathop{C}}\,{{H}_{2}}I \\ \underset{\,||}{\mathop{C}}\,H\,\,\, \\ C{{H}_{2}} \\ \end{matrix}}}\,\]
(2) Properties : It is a colourless liquid. It boils at 103.1°C.The halogen atom in allyl iodide is quite reactive. The p-orbital of the halogen atom does not interact with p-molecular orbital of the double bond because these are separated by a saturated \[s{{p}^{3}}\]-hybridized carbon atom. Thus, the halogen atom in allyl halides can be easily replaced and the reactions of allyl halides are similar to the reaction of alkyl halides.
In terms of valence bond approach, the reactivity of halogen atom is due to ionisation to yield a carbonium ion which can stabilize by resonance as shown below,
\[C{{H}_{2}}=CH-C{{H}_{2}}I\xrightarrow{{}}\]\[[C{{H}_{2}}=CH-\overset{+}{\mathop{C}}\,{{H}_{2}}\overset{{}}{\longleftrightarrow}\overset{+}{\mathop{C}}\,{{H}_{2}}-CH=C{{H}_{2}}]+{{I}^{-}}\]
Substitution reactions : Nucleophilic substitution reactions occur,
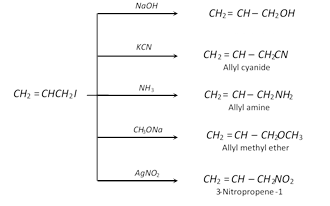
Addition reactions : Electrophilic addition reactions take place in accordance to Markownikoff's rule.
\[C{{H}_{2}}=CH-C{{H}_{2}}I+B{{r}_{2}}\xrightarrow{{}}\underset{\text{1,2}-\text{Dibromo-3-iodopropane}}{\mathop{C{{H}_{2}}Br\cdot CHBr\cdot C{{H}_{2}}I}}\,\]
\[C{{H}_{2}}=CH-C{{H}_{2}}I+HBr\xrightarrow{{}}\underset{\text{2}-\text{Bromo-1-iodopropane}}{\mathop{C{{H}_{3}}CHBrC{{H}_{2}}I}}\,\]
Allyl iodide is widely used in organic synthesis.
You need to login to perform this action.
You will be redirected in
3 sec
