Resonance Effect Or Mesomeric Effect
Category : JEE Main & Advanced
(1) The effect in which \[\pi \] electrons are transferred from a multiple bond to an atom, or from a multiple bond to a single covalent bond or lone pair (s) of electrons from an atom to the adjacent single covalent bond is called mesomeric effect or simply as M-effect. In case of the compound with conjugated system of double bonds, the mesomeric effect is transmitted through whole of the conjugated system and thus the effect may better be known as conjugative effect.
(2) Groups which have the capacity to increase the electron density of the rest of the molecule are said to have \[+M\] effect. Such groups possess lone pairs of electrons. Groups which decrease the electron density of the rest of the molecule by withdrawing electron pairs are said to have \[-M\] effect, e.g.,
(a) The groups which donate electrons to the double bond or to a conjugated system are said to have \[+M\] effect or \[+R\] effect.
\[+M\] effect groups :
\[-Cl,\,-Br,\,-I,\,-\overset{.\,\,.}{\mathop{N}}\,{{H}_{2}},\,-N{{R}_{2}},-OH,-OR,-SH,-OC{{H}_{3}},-\overset{.\,\,.}{\mathop{\underset{.\,\,.}{\mathop{S}}\,}}\,R\]
(b) The groups which withdraw electrons from the double bond or from a conjugated system towards itself due to resonance are said to have \[-M\] effect or \[-R\] effect.
\[-M\] effect groups :
\[-N{{O}_{2}},-C\equiv N,\,-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-,-CHO,-COOH,-S{{O}_{3}}H\]
(3) The inductive and mesomeric effects, when present together, may act in the same direction or oppose each other. The mesomeric effect is more powerful than the former. For example, in vinyl chloride due to \[-I\] effect the chlorine atom should develop a negative charge but on account of mesomeric effect it has positive charge.
![]()
Application of mesomeric effect : It explains,
(1) Low reactivity of aryl and vinyl halides,
(2) The acidic nature of carboxylic acids,
(3) Basic character comparison of ethylamine and aniline,
(4) The stability of some free radicals, carbocations and carbanions.
Difference between Resonance and Mesomerism : Although both resonance and mesomerism represent the same phenomenon, they differ in the following respect : Resonance involves all types of electron displacements while mesomerism is noticeable only in those cases where a multiple bond is in conjugation with a multiple bond or lone pair of electron.
Example :
(i) ![]()
(ii) 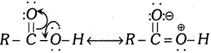
Both (i) and (ii) are the examples of mesomerism and resonance effect. Let us consider the following example.
![]()
Such an electron displacement is the example of resonance only (not the mesomerism).
You need to login to perform this action.
You will be redirected in
3 sec
