Elimination Reactions
Category : JEE Main & Advanced
Elimination reactions are formally the reverse of addition reactions and involve the removal of the two groups (Generally, one being a proton) from one or two carbon atoms of a molecule to form an unsaturated linkage or centre.
Elimination reaction is given by those compounds which have a nucleophilic group as leaving group,
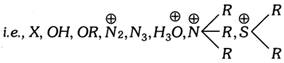
Elimination reactions are generally endothermic and take place on heating.
Elimination reactions are classified into two general types,
(I) \[\alpha -\]elimination reactions or 1, 1-elimination reactions.
(II) \[\beta -\]elimination reaction or 1, 2-elimination reactions.
(I) \[\alpha -\]elimination reactions or 1,1-elimination reactions: A reaction in which both the groups or atoms are removed from the same carbon of the molecule is called \[\alpha -\]elimination reaction. This reaction is mainly given by gem dihalides and gem trihalides having at least one \[\alpha -\]hydrogen.
\[CH{{X}_{3}}\xrightarrow{\text{Alc}\text{.}\,\,KOH\text{/}\Delta }\overset{.\,.\,\,\,\,}{\mathop{C{{X}_{2}}}}\,+\overset{\Theta }{\mathop{X}}\,+\overset{\oplus }{\mathop{H}}\,\]
Product of the reaction is halocarbenes or dihalocarbenes. which are key intermediates in a wide variety of chemical and photochemical reactions.
(II) \[\beta -\]elimination reactions or 1, 2-elimination reactions: Consider the following reactions,
\[C{{H}_{3}}-\underset{\beta }{\mathop{C{{H}_{2}}}}\,-\underset{\alpha }{\mathop{C{{H}_{2}}}}\,-L\to C{{H}_{3}}-CH=C{{H}_{2}}+\overset{\oplus }{\mathop{H}}\,+\overset{\Theta }{\mathop{L}}\,\]
A reaction in which functional group (i.e., leaving group) is removed from \[\alpha -\]carbon and other group (Generally hydrogen atom) from the \[\beta -\]carbon is called \[\beta -\]elimination reaction. In this reaction there is loss of two \[\sigma \] bonds and gain of one \[\pi \] bond. Product of the reaction is generally less stable than the reactant.
(1) Types of \[\beta -\]elimination reactions : In analogy with substitution reactions, \[\beta -\] elimination reactions are divided into three types:
(i) \[{{E}_{1}}\] (Elimination unimolecular) reaction, (ii) \[{{E}_{2}}\] (Elimination bimolecular) reaction and (iii) \[{{E}_{1\,cb}}\] (Elimination unimolecular conjugate base) reaction
(i) \[{{E}_{1}}\] (Elimination unimolecular) reaction : Consider the following reaction,

(a) Reaction velocity depends only on the concentration of the substrate; thus reaction is unimolecular reaction.
Rate \[\propto \] [Substrate]
(b) Product formation takes place by the formation of carbocation as reaction intermediate \[(RI)\].
(c) Since reaction intermediate is carbocation, rearrangement is possible in \[{{E}_{1}}\] reaction.
(d) Reaction is carried out in the presence of polar protic solvent.
(e) The \[{{E}_{1}}\] reaction occurs in two steps,
Step 1.
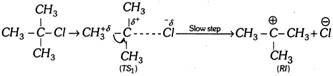
Step 2.
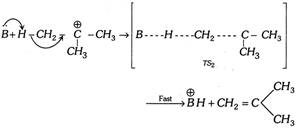
(ii) \[{{E}_{2}}\] (Elimination bimolecular) reaction : Consider the following reaction,
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-Br\underset{\Delta }{\mathop{\xrightarrow{Base\,(B)}}}\,C{{H}_{3}}-CH=C{{H}_{2}}+\overset{\oplus }{\mathop{H}}\,+\overset{\Theta }{\mathop{Br}}\,\]
(a) Reaction velocity depends only on the concentration of the substrate and the base used; thus reaction is bimolecular reaction. Rate \[\propto \][Substrate] [Base]
(b) Since the reaction is a bimolecular reaction, the product formation will take place by formation of transition state (TS).
(c) Rearrangement does not take place in \[{{E}_{2}}\] reaction but in case of allylic compound rearrangement is possible.
(d) Reaction is carried out in the presence of polar aprotic solvent.
(e) The \[{{E}_{2}}\] reaction occurs in one step,
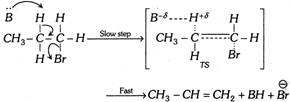
(2) Orientation in \[\mathbf{\beta -}\]elimination reactions : If substrate is unsymmetrical, then this will give more than one product. Major product of the reaction can be known by two emperical rules.
(i) Saytzeff rule : According to this rule, major product is the most substituted alkene i.e., major product is obtained by elimination of \[\overset{\oplus }{\mathop{H}}\,\] from that \[\beta -\]carbon which has the least number of hydrogen. Product of the reaction in this case is known as Saytzeff product.
\[\underset{\,\,\ \ \ \ \ \ \ C{{H}_{3}}}{\mathop{C{{H}_{3}}-\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{{{\beta }_{2}}}{\mathop{CH}}\,}}\,}}\,-\,\overset{Cl\,}{\mathop{\overset{|\,\,\,\,\,}{\mathop{\underset{\alpha \,\,\,\,\,}{\mathop{CH\,}}\,}}\,}}\,-\underset{{{\beta }_{1}}}{\mathop{C{{H}_{3}}}}\,\underset{-HCl}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH/\Delta }}}\,\underset{\text{Saytzeff product}}{\mathop{C{{H}_{3}}\underset{C{{H}_{3}}\ \ \ }{\mathop{\underset{|\,\ \ \ \ \ \ \ \ \,}{\mathop{-C=CH}}\,}}\,-C{{H}_{3}}}}\,\]
(ii) Hofmann rule : According to this rule, major product is always least substituted alkene i.e., major product is formed from \[\beta -\]carbon which has maximum number of hydrogen. Product of the reaction in this case is known as Hofmann product.
\[\begin{matrix}\ \ \ \ \ C{{H}_{3}} \\C{{H}_{3}}-\underset{|\ \ \ }{\mathop{\overset{|\ \ \ }{\mathop{C-}}\,}}\,\underset{{{\beta }_{2}}}{\mathop{C{{H}_{2}}}}\, \\ \ \ \ \ \ C{{H}_{3}} \\ \end{matrix}\overset{Br\,\,}{\mathop{\overset{|\,\,\,\,\,}{\mathop{\underset{\alpha }{\mathop{CH}}\,}}\,}}\,-\underset{{{\beta }_{1}}}{\mathop{C{{H}_{3}}}}\,\xrightarrow{\text{Alc}\text{.}\,KOH/\Delta }\,\,\underset{\text{Hofmannproduct}}{\mathop{C{{H}_{3}}\begin{matrix}C{{H}_{3}}\ \ \\-\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,-C{{H}_{2}} \\C{{H}_{3}}\ \ \\\end{matrix}-CH=C{{H}_{2}}}}\,\]
(3) Examples of \[\mathbf{\beta -}\]elimination reactions
(i) Dehydrohalogenation is removal of HX from alkyl halides with alcoholic KOH or \[KN{{H}_{2}}\] or ter- BuOK (Potassium tertiary butoxide) and an example of \[\alpha -\beta \] elimination,
e.g.,\[C{{H}_{3}}-C{{H}_{2}}X\underset{(-HX)}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH}}}\,\underset{\text{Ethene}}{\mathop{{{H}_{2}}C=C{{H}_{2}}}}\,\];
\[C{{H}_{3}}-\underset{X}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\underset{(-HX)}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH}}}\,\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,\]
\[C{{H}_{3}}-C{{H}_{2}}-\underset{X}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\underset{(-HX)}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH}}}\,\underset{\text{2- Butene (Major)}}{\mathop{C{{H}_{3}}-CH=CH-C{{H}_{3}}}}\,+\]\[\underset{\text{1- Butene (Minor)}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-CH=C{{H}_{2}}}}\,\]
(ii) Dehydration of alcohol is another example of elimination reaction. When acids like conc. \[{{H}_{2}}S{{O}_{4}}\] or \[{{H}_{3}}P{{O}_{4}}\] are used as dehydrating agents, the mechanism is \[{{E}_{1}}\]. The proton given by acid is taken up by alcohol.
Dehydration is removal of \[{{H}_{2}}O\] from alcohols,
e.g.,
\[C{{H}_{3}}-C{{H}_{2}}-OH\underset{(-{{H}_{2}}O)}{\mathop{\xrightarrow{\text{Conc}\text{. }{{H}_{2}}S{{O}_{4}},\,170{}^\circ C}}}\,{{H}_{2}}C=C{{H}_{2}}\]
\[\underset{\text{Propan-1-ol}}{\mathop{C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-OH}}\,\underset{(-{{H}_{2}}O)}{\mathop{\xrightarrow{\text{Conc}\text{. }{{H}_{2}}S{{O}_{4}},\,170{}^\circ C}}}\,\underset{\text{Propene}}{\mathop{C{{H}_{3}}-CH=C{{H}_{2}}}}\,\]
\[C{{H}_{3}}-C{{H}_{2}}-\underset{OH}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}\] and so
(iii) Dehalogenation : It is removal of halogens, e.g., \[\]\[\underset{\text{Ethylene}\,\text{bromide}}{\mathop{\underset{Br\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,-\underset{Br\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,}}\,+Zn\,\text{dust}\underset{\text{(-ZnB}{{\text{r}}_{\text{2}}})}{\mathop{\xrightarrow{\text{in}\,\text{C}{{\text{H}}_{\text{3}}}OH,\,\text{heat}}}}\,\underset{\text{Ethylene}}{\mathop{{{H}_{2}}C=C{{H}_{2}}}}\,\]
(iv) Dehydrogenation : It is removal of hydrogen, e.g., \[\underset{\text{Isopropyl alcohol}}{\mathop{C{{H}_{3}}-\underset{OH}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}}}\,\underset{(-{{H}_{2}})}{\mathop{\xrightarrow{Cu,\,300{}^\circ C}}}\,\,\,\underset{\text{Acetone}}{\mathop{C{{H}_{3}}-\underset{O}{\mathop{\underset{|\,|}{\mathop{C}}\,}}\,-C{{H}_{3}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
