Extended or long form of periodic table
Category : JEE Main & Advanced
Modern periodic table is also called long form of the periodic table or Bohr?s table. In this table, the elements are arranged in order of their increasing atomic number. It consists of 4 blocks (s, p, d and f), 18 groups numbered from 1 to 18 and 7 periods numbered from 1 to 7.
Blocks : The periodic table is divided into four main blocks (s, p, d and f) depending upon the subshell to which the valence electron enters into.
(1) Elements of group 1 and 2 constitute s-Block.
(2) Elements of group 13, 14, 15, 16, 17, 18 constitute p-Block
(3) Elements of group 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 constitute d-Block
(4) The f-Block elements comprise two horizontal rows placed at the bottom of the periodic table to avoid its unnecessary expansion.
Elements of s- and p-blocks are called normal or representative elements, those of d-block are called transition elements while the f-block elements are called inner transition elements.
Groups : The 18 vertical columns are called groups. The elements belonging to a particular group is known as a family and is usually named after the first number. Apart from this some of the groups are given typical names as examplified beneath,
(1) Elements of group 1 are called Alkali-Metals.
(2) Elements of group 2 are called Alkaline Earths.
(3) Elements of group 3 are called Pnicogens.
(4) Elements of group 16 are called Chalcogens.
(5) Elements of group 17 are called Halogens.
(6) Elements of group 18 are called Noble Gases or Aerogens.
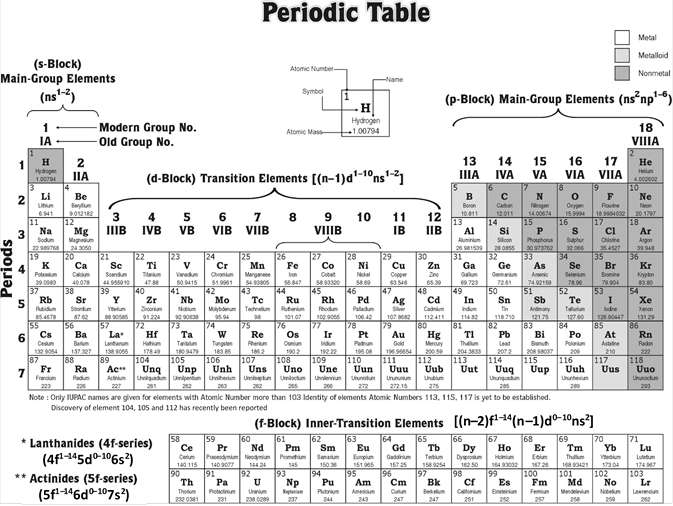
All the other groups are named after the first member of each group.
Periods : The horizontal rows are called periods. There are seven periods in the long form of the periodic table,
(1) Ist period \[_{1}H{{\to }_{2}}He)\] contains 2 elements. It is the shortest period.
(2) 2nd period \[{{(}_{3}}Li{{\to }_{10}}Ne)\] and 3rd period \[{{(}_{11}}Na{{\to }_{18}}Ar)\] contains 8 elements each. These are short periods.
(3) 4th period \[{{(}_{19}}K{{\to }_{36}}Kr)\] and 5th period \[{{(}_{37}}Rb{{\to }_{54}}Xe)\] contains 18 elements each. These are long periods.
(4) 6th period \[{{(}_{55}}Cs{{\to }_{86}}Ra)\] consists of 32 elements and is the longest period.
(5) 7th period starting with \[_{87}Fr\] is incomplete and consists of 19 elements.
You need to login to perform this action.
You will be redirected in
3 sec
