Acid derivatives
Category : JEE Main & Advanced
The compounds which are obtained by replacing the \[-OH\] of the carboxylic group by other atoms or groups such as \[{{X}^{-}},\,-N{{H}_{2}}\], – OR and \[O-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-R\] are known as acid derivatives.
| Group replacing \[-OH\] | Name | Structure |
| \[(X=F,\,Cl,\,Br,\,I)\] | Acyl halide | \[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-X\] |
| \[-N{{H}_{2}}\] | Amide | \[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-N{{H}_{2}}\] |
| \[-O{R}'\] | ester | \[\underset{\text{(}{R}'\,\text{may be }R\text{)}}{\mathop{R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O{R}'}}\,\] |
| \[-OOCR\] | anhydride | \[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-R\] |
Reactivity
Acyl derivatives are characterised by nucleophilic substitution reactions.
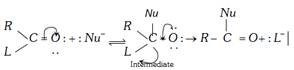
\[(L=X,\,N{{H}_{2}},\,O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-R\,\text{or}\,OR)\]
The relative reactivities of various acyl compounds have been found to be in the following order:
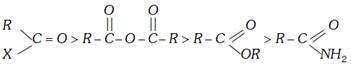
Out of acid halides, the acid chlorides are more important ones.
The overall order of reactivity can be accounted for in terms of the following three factors:
(i) Basicity of the leaving group (ii) Resonance effects and (iii) Inductive effects.
(i) Basicity of the leaving group : Weaker bases are good leaving groups. Hence, the acyl derivatives with weaker bases as leaving groups are more reactive. Chloride ion is the weakest base while –\[N{{H}_{2}}\] is the strongest base. Thus, acyl chlorides are most reactive and amides are least reactive.
(ii) Resonance effect : The leaving group in each case has an atom with lone pair of electrons adjacent to the carbonyl group. The compound exists, therefore, as a resonance hybrid.
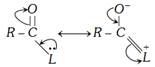
This makes the molecule more stable. The greater the stabilization, the smaller is the reactivity of the acyl compound.
However, acyl chlorides are least affected by resonance. Due to lower stabilization, the acid chlorides are more reactive as the loss of \[-Cl\] is easier. Greater stabilization is achieved by resonance in esters and amides and thus, they are less reactive.
(iii) Inductive effect : Higher the \[-I\] effect, more reactive is the acyl compound. Inductive effect of oxygen in ester is greater than nitrogen in amide, hence ester is more reactive than an amide.
![]()
where R may be alkyl or aryl group.
(1) Methods of Preparation
(i) From carboxylic acid :
\[RCOOH+PC{{l}_{5}}\to RCOCl+POC{{l}_{3}}+HCl\]
\[3RCOOH+PC{{l}_{3}}\to 3RCOCl+{{H}_{3}}P{{O}_{3}}\]
(ii) Industrial method : By distilling anhydrous sodium acetate
\[3C{{H}_{3}}COONa+PC{{l}_{3}}\xrightarrow{\text{heat}}3C{{H}_{3}}COCl+N{{a}_{3}}P{{O}_{3}}\]
\[\underset{\text{Sodium acetate}}{\mathop{2C{{H}_{3}}COONa}}\,+POC{{l}_{3}}\xrightarrow{\text{heat}}\underset{\text{Acetyl chloride}}{\mathop{2C{{H}_{3}}COCl}}\,+NaP{{O}_{3}}+NaCl\]
\[\underset{\text{Calcium acetate}}{\mathop{{{(C{{H}_{3}}COO)}_{2}}Ca}}\,+\underset{\begin{smallmatrix} \text{Sulphuryl } \\ \text{chloride} \end{smallmatrix}}{\mathop{S{{O}_{2}}C{{l}_{2}}}}\,\xrightarrow{\text{heat}}\underset{\text{Acetyl chloride}}{\mathop{2C{{H}_{3}}COCl}}\,+CaS{{O}_{4}}\]
(iii) With thionyl chloride :
\[RCOOH+SOC{{l}_{2}}\to RCOCl+S{{O}_{2}}+HCl\]
This is the best method because \[S{{O}_{2}}\] and HCl are gases and easily escape leaving behind acyl chloride.
(2) Physical properties : The lower acyl chloride are mobile, colourless liquid while the higher members are coloured solids.
Acyl chloride have very pungent, irritating order and are strong lachrymators (tears gases)
They fume in air due to the formation of hydrochloric acid by hydrolysis.
They are readily soluble in most of the organic solvent. Acyl chloride don't form intermolecular hydrogen bonding. Therefore, their boiling points are lower than those of their parent acids.
(3) Chemical properties
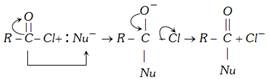
\[C{{l}^{-}}+{{H}^{+}}\to HCl\]
(i) Hydrolysis : \[\underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}COCl}}\,+HOH\to \underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+HCl\]
\[\underset{\text{Benzoyl chloride}}{\mathop{{{C}_{6}}{{H}_{5}}COCl}}\,+{{H}_{2}}O\to \underset{\text{Benzoic acid}}{\mathop{{{C}_{6}}{{H}_{5}}COOH}}\,+{{H}_{2}}O\]
(ii) Reaction with alcohols (alcoholysis)
\[C{{H}_{3}}COCl+C{{H}_{3}}C{{H}_{2}}OH\to \underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{2}}C{{H}_{3}}}}\,+HCl\]
\[\underset{\text{Benzoyl chloride}}{\mathop{{{C}_{6}}{{H}_{5}}COCl}}\,+\underset{\text{Ethyl alcohol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\underset{\text{Pyridine}}{\mathop{\xrightarrow{\text{aq}\,NaOH\,\text{or}}}}\,\underset{\text{Ethyl benzoate}}{\mathop{{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}}}\,+HCl\]
This reaction is called Schotten Baumann reaction.
(iii) Reaction with salts of carboxylic acid
\[C{{H}_{3}}COCl+C{{H}_{3}}CO{{O}^{-}}N{{a}^{+}}\xrightarrow{\text{Pyridine}}\underset{\text{Acetic anhydride}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}}}\,\]
(iv) Reaction with benzene (acylation) : This reaction is called friedel craft reaction.
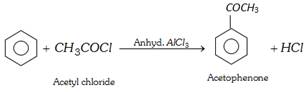
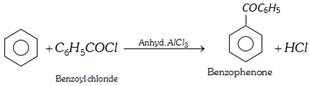
(v) Reaction with ammonia or amines :
\[\underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}COCl}}\,+2N{{H}_{3}}\to \underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+N{{H}_{4}}Cl\]
\[{{C}_{6}}{{H}_{5}}COCl+2N{{H}_{3}}\to \underset{\text{Benzamide}}{\mathop{{{C}_{6}}{{H}_{5}}CON{{H}_{2}}}}\,+N{{H}_{4}}Cl\]
However, acyl chlorides react with amines to form substituted amides.
\[C{{H}_{3}}COCl+{{H}_{2}}N{{C}_{2}}{{H}_{5}}\to \underset{\text{N-Ethyl acetamide}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-NH-{{C}_{2}}{{H}_{5}}}}\,\]
\[C{{H}_{3}}COCl+{{({{C}_{2}}{{H}_{5}})}_{2}}NH\to \underset{\text{N, N-Diethyl acetamide}}{\mathop{C{{H}_{3}}CON{{({{C}_{2}}{{H}_{5}})}_{2}}+HCl}}\,\]
(vi) Reduction :
\[C{{H}_{3}}COCl\underset{NaB{{H}_{4}}}{\mathop{\xrightarrow{LiAl{{H}_{4}}\,\text{or}}}}\,\overset{{}}{\mathop{\underset{\text{Ethanol (Primary alcohol)}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,}}\,\]
\[C{{H}_{3}}COCl\underset{{}}{\mathop{+{{H}_{2}}\xrightarrow{Pd/BaS{{O}_{4}}}}}\,C{{H}_{3}}CHO+HCl\]
This reaction is called Rosenmund reaction.
(vii) Reaction with organocadmium compounds (formation of ketones)
\[2C{{H}_{3}}COCl+\underset{\begin{smallmatrix} \text{Dimethyl} \\ \text{Cadmium} \end{smallmatrix}}{\mathop{{{(C{{H}_{3}})}_{2}}Cd}}\,\to \underset{\text{Acetone}}{\mathop{2C{{H}_{3}}COC{{H}_{3}}}}\,+CdC{{l}_{2}}\]
\[2{{C}_{6}}{{H}_{5}}COCl+{{(C{{H}_{3}})}_{2}}Cd\to \underset{\text{Acetophenone}}{\mathop{2{{C}_{6}}{{H}_{5}}COC{{H}_{3}}}}\,+CdC{{l}_{2}}\]
(viii) Reaction with diazomethane
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-Cl+\underset{\text{Diazomethane}}{\mathop{2\bar{C}{{H}_{2}}-\overset{+}{\mathop{N}}\,}}\,\equiv N\to \underset{\text{Diazoacetone}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-\overset{-}{\mathop{C}}\,H-\overset{+}{\mathop{N}}\,}}\,\equiv N\]\[\underset{(-{{N}_{2}})}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,C{{H}_{3}}C{{H}_{2}}\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-OH\]
(ix) Reaction with water
\[C{{H}_{3}}COCl\xrightarrow{AgN{{O}_{3}}/{{H}_{2}}O}C{{H}_{3}}COOH+AgCl+HN{{O}_{3}}\]
(x) Reaction with chlorine
\[C{{H}_{3}}COCl+C{{l}_{2}}\xrightarrow{\text{Red }P}\underset{\text{Mono-}\alpha \text{-chloroacetyl chloride}}{\mathop{Cl-C{{H}_{2}}-CO-Cl+HCl}}\,\]
(xi) Reaction with Grignard reagent
![]()
(xii) Reaction with KCN
\[C{{H}_{3}}COCl+KCN\to \underset{\text{Acetyl cyanide}}{\mathop{C{{H}_{3}}COCN}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Pyruvic acid}}{\mathop{C{{H}_{3}}COCOOH}}\,\]
(xiii) Reaction with Salicylic acid
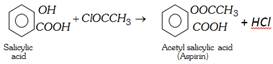
(xiv) Reaction with ether
\[C{{H}_{3}}COCl+\underset{\text{Diethyl ether}}{\mathop{{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}}}\,\underset{\text{anhy}\text{.}}{\mathop{\xrightarrow{ZnC{{l}_{2}}}}}\,\]\[\underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+\underset{\text{Ethyl chloride}}{\mathop{{{C}_{2}}{{H}_{5}}Cl}}\,\]
(xv) Reaction with sodium peroxide (Peroxide formation)
\[\underset{\text{Acetyl chloride}}{\mathop{2C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-Cl}}\,+\overset{+}{\mathop{N}}\,a\overset{-}{\mathop{O}}\,-\overset{-}{\mathop{O}}\,\overset{+}{\mathop{N}}\,a\to \underset{\text{Acetyl peroxide}}{\mathop{C{{H}_{3}}\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O-O-}}\,\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{_{3}}}+2NaCl\]
(xvi) Reaction with hydroxylamine and hydrazine
\[C{{H}_{3}}COCl+\underset{\begin{smallmatrix} \text{Hydroxyl}\,\,\,\,\,\,\, \\ \text{amine} \end{smallmatrix}}{\mathop{{{H}_{2}}NOH}}\,\to \underset{\begin{smallmatrix} \text{Acetyl hydroxylamine} \\ \,\,\,\,\text{(hydroxamic acid)} \end{smallmatrix}}{\mathop{C{{H}_{3}}CONHOH}}\,+HCl\]
\[C{{H}_{3}}COCl+\underset{\text{Hydrazine}}{\mathop{{{H}_{2}}NN{{H}_{2}}}}\,\to \underset{\text{Acetyl hydrazine}}{\mathop{C{{H}_{3}}CONHN{{H}_{2}}}}\,+HCl\]
(4) Uses
(i) As an acetylating agent.
(ii) In the estimation and determination of number of hydroxyl and amino groups.
(iii) In the preparation of acetaldehyde, acetic anhydride, acetamide, acetanilide, aspirin, acetophenone etc.
![]()
where, \[R=-C{{H}_{3}},\,-C{{H}_{2}}C{{H}_{3}},\,-{{C}_{6}}{{H}_{5}}\]
(1) Methods of preparation
(i) Ammonolysis of acid derivatives
\[C{{H}_{3}}COCl+2N{{H}_{3}}\to \underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+N{{H}_{4}}Cl\]
\[{{(C{{H}_{3}}CO)}_{2}}O+2N{{H}_{3}}\to \underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+\underset{\text{Amm}\text{. acetate}}{\mathop{C{{H}_{3}}COON{{H}_{4}}}}\,\]
\[\underset{\text{Benzoyl chloride}}{\mathop{{{C}_{6}}{{H}_{5}}COCl}}\,+N{{H}_{3}}\to \underset{\text{Benzamide}}{\mathop{{{C}_{6}}{{H}_{5}}CON{{H}_{2}}}}\,+HCl\]
(ii) From ammonium salts of carboxylic acids (Laboratory Method)
\[C{{H}_{3}}COON{{H}_{4}}\xrightarrow{\text{Heat}}\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+{{H}_{2}}O\]
q Ammonium acetate is always heated in presence of glacial acetic acid to avoid the side product (\[C{{H}_{3}}COOH)\].
(iii) By partial hydrolysis of alkyl cyanide :
\[C{{H}_{3}}C\equiv N\underset{{{H}_{2}}O/O{{H}^{-}}}{\mathop{\xrightarrow{\text{Conc}\text{. }HCl}}}\,\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\]
(iv) By heating carboxylic acid and urea
\[{{H}_{2}}N-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-N{{H}_{2}}+R-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-OH\xrightarrow{\text{heat}}\underset{\text{Amide}\,\,\,\,\,}{\mathop{R-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-N{{H}_{2}}}}\,+C{{O}_{2}}+N{{H}_{3}}\]
(2) Physical properties
(i) Physical state : Formamide is a liquid while all other amides are solids.
(ii) Boiling points : Amides have high boiling points than the corresponding acids.
Acetamide Boiling points 494 K
Acetic Acid Boiling points 391 K
Benzamide Boiling points 563 K
Benzoic acid Boiling points 522 K
The higher boiling points of amides is because of intermolecular hydrogen bonding
\[..........H-\overset{H}{\mathop{\overset{|}{\mathop{N}}\,}}\,-\overset{R}{\mathop{\overset{|}{\mathop{C}}\,}}\,=O.........H-\overset{H}{\mathop{\overset{|}{\mathop{N}}\,}}\,-\overset{R}{\mathop{\overset{|}{\mathop{C}}\,}}\,=O.........H-\overset{H}{\mathop{\overset{|}{\mathop{N}}\,}}\,-\overset{R}{\mathop{\overset{|}{\mathop{C}}\,}}\,=O\]
(iii) Solubility : The lower members of amide family are soluble in water due to the formation of hydrogen bonds with water.
(3) Chemical properties
(i) Hydrolysis
\[C{{H}_{3}}CON{{H}_{2}}+{{H}_{2}}O\xrightarrow{\text{Slowly}}C{{H}_{3}}COOH+N{{H}_{3}}\]
\[C{{H}_{3}}CON{{H}_{2}}+{{H}_{2}}O+HCl\xrightarrow{\text{Rapidly}}C{{H}_{3}}COOH\]\[+N{{H}_{4}}Cl\]
\[C{{H}_{3}}CON{{H}_{2}}+NaOH\xrightarrow{\text{Far more rapidly}}C{{H}_{3}}COONa+N{{H}_{3}}\]
(ii) Amphoteric nature (Salt formation)
It shows feebly acidic as well as basic nature.
\[C{{H}_{3}}CON{{H}_{2}}+HCl(\text{conc}\text{.})\to \underset{\begin{smallmatrix} \text{Acetamide hydrochloride} \\ \text{(only stable in aqueous solution)} \end{smallmatrix}}{\mathop{C{{H}_{3}}CON{{H}_{2}}.HCl}}\,\]
\[\underset{\text{Acetamide}}{\mathop{2C{{H}_{3}}CON{{H}_{2}}}}\,+\underset{\begin{smallmatrix} \text{Mercuric } \\ \,\,\text{Oxide}\,\, \end{smallmatrix}}{\mathop{HgO}}\,\to \underset{\text{Mercuric acetamide}\,\,\,\,\,\,\,\,\,\,\,}{\mathop{{{(C{{H}_{3}}CONH)}_{2}}Hg+{{H}_{2}}O}}\,\]
\[C{{H}_{3}}CON{{H}_{2}}+Na\xrightarrow{\text{Ether}}\underset{\text{Sodium acetamide}}{\mathop{C{{H}_{3}}CONHNa}}\,+\frac{1}{2}{{H}_{2}}\]
(iii) Reduction
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+4[H]\xrightarrow{LiAl{{H}_{4}}}\underset{\text{Ethylamine}}{\mathop{C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}}}\,+{{H}_{2}}O\]
\[\underset{\text{Benzamide}}{\mathop{{{C}_{6}}{{H}_{5}}CON{{H}_{2}}}}\,+4[H]\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}\underset{\text{Benzylamine}}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}N{{H}_{2}}}}\,+{{H}_{2}}O\]
(iv) Dehydration
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\underset{\text{heat}}{\mathop{\xrightarrow{{{P}_{2}}{{O}_{5}}}}}\,\underset{\text{Methyl cyanide}}{\mathop{C{{H}_{3}}C\equiv N}}\,+{{H}_{2}}O\]
\[\underset{\text{Benzamide}}{\mathop{{{C}_{6}}{{H}_{5}}CON{{H}_{2}}}}\,\underset{\text{heat}}{\mathop{\xrightarrow{{{P}_{2}}{{O}_{5}}}}}\,\underset{\text{Phenyl cyanide}}{\mathop{{{C}_{6}}{{H}_{5}}C\equiv N}}\,+{{H}_{2}}O\]
\[{{C}_{6}}{{H}_{5}}CON{{H}_{2}}\underset{{}}{\mathop{\xrightarrow{SOC{{l}_{2}}}}}\,\underset{\text{Phenyl cyanide}}{\mathop{{{C}_{6}}{{H}_{5}}C\equiv N}}\,\]
(v) Reaction with nitrous acid
\[C{{H}_{3}}CON{{H}_{2}}+HONO\xrightarrow{NaN{{O}_{2}}/HCl}\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+{{N}_{2}}\] \[+{{H}_{2}}O\]
\[{{C}_{6}}{{H}_{5}}CON{{H}_{2}}+HONO\xrightarrow{NaN{{O}_{2}}/HCl}\underset{\text{Benzoic}\,\text{ acid}}{\mathop{{{C}_{6}}{{H}_{5}}COOH}}\,\] \[+{{N}_{2}}+{{H}_{2}}O\]
(vi) Hofmann bromamide reaction or Hofmann degradation : This is an important reaction for reducing a carbon atom from a compound, i.e., \[-CON{{H}_{2}}\] is changed to \[-N{{H}_{2}}\] group.
\[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,\underset{NaOH\,\text{or }KOH}{\mathop{\xrightarrow{B{{r}_{2}}}}}\,\underset{\text{Methyl amine (p-)}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,\]
This reaction occurs is three steps:
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-N{{H}_{2}}+B{{r}_{2}}+KOH\to \underset{\text{Acetobromamide}}{\mathop{C{{H}_{3}}CONHBr}}\,+KBr+{{H}_{2}}O\]
\[C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-NHBr+KOH\to \underset{\text{Methyl isocyanate}}{\mathop{C{{H}_{3}}NCO}}\,+KBr+{{H}_{2}}O\]
\[C{{H}_{3}}NCO+2KOH\to \underset{\text{Methyl amine}}{\mathop{C{{H}_{3}}N{{H}_{2}}}}\,+{{K}_{2}}C{{O}_{3}}\]
\[\overline{C{{H}_{3}}CON{{H}_{2}}+B{{r}_{2}}+4KOH\to C{{H}_{3}}N{{H}_{2}}+2KBr+{{K}_{2}}C{{O}_{3}}+2{{H}_{2}}O}\]
q In this reaction a number of intermediates have been isolated; N-bromamides, salts of these bromamides ; Isocyanates, RNCO.
q Nitrene rearranges to form isocyanate.
(vii) Action with alcohol :
\[C{{H}_{3}}CON{{H}_{2}}+C{{H}_{3}}OH\underset{{{70}^{o}}C}{\mathop{\xrightarrow{HCl}}}\,\underset{\text{methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,+N{{H}_{4}}Cl\]
(viii) Reaction with grignard reagent
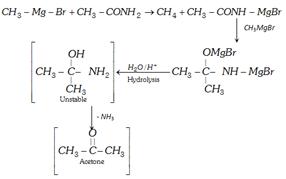
(4) Uses
(i) In organic synthesis. The compounds like methyl cyanide, Methylamine and ethylamine can be prepared.
(ii) In leather tanning and paper industry.
(iii) As a wetting agent and as soldering flux.
Amides such as dimethyl formamide (DMF), dimethyl acetamide (DMA) are used as solvents for organic and inorganic compounds.
Esters, \[R-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-OR\]
These are the most important class of acid derivatives and are widely distributed in nature in plants, fruits and flowers.
(1) Methods of preparation
(i) From carboxylic acid [Esterification] : Laboratory method.
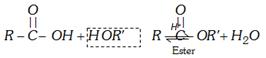
\[\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+\underset{\text{Diazomethane}}{\mathop{C{{H}_{2}}{{N}_{2}}}}\,\,\xrightarrow{\text{Ether}}\,\underset{\text{Methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,+{{N}_{2}}\]
\[\underset{\text{Benzoic acid}}{\mathop{{{C}_{6}}{{H}_{5}}COOH}}\,+\underset{\text{Diazomethane}}{\mathop{C{{H}_{2}}{{N}_{2}}}}\,\,\xrightarrow{\text{Ether}}\,\underset{\text{Methyl benzoate}}{\mathop{{{C}_{6}}{{H}_{5}}COOC{{H}_{3}}}}\,+{{N}_{2}}\]
(ii) From acid chloride or acid anhydrides
![]()
![]()
![]()
(iii) From alkyl halide :
\[\underset{\text{Ethyl bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,+\underset{\text{Silver acetate}}{\mathop{C{{H}_{3}}COOAg}}\,\to \underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+AgBr\]
(iv) From ether :
\[\underset{\text{Methoxy methane}}{\mathop{C{{H}_{3}}-O-C{{H}_{3}}}}\,+CO\underset{350K}{\mathop{\xrightarrow{B{{F}_{3}}}}}\,\underset{\text{Methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,\]
(v) From Tischenko reaction :
\[C{{H}_{3}}-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-H+O=\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,-C{{H}_{3}}\xrightarrow{Al{{(O{{C}_{2}}{{H}_{5}})}_{3}}}C{{H}_{3}}-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-O{{C}_{2}}{{H}_{5}}\]
(2) Physical properties
(i) Physical state and smell : Esters are colourless liquids (or solids) with characteristic fruity smell. Flavours of some of the esters are listed below :
| Ester | Flavour | Ester | Flavour |
| Amyl acetate | Banana | Isobutyl formate | Raspberry |
| Benzyl acetate | Jasmine | Ethyl butyrate | Pineapple |
| Amyl butyrate | Apricot | Octyl acetate | Orange |
(ii) Solubility : They are sparingly soluble in water but readily soluble in organic solvents such as alcohol, ether etc.
(iii) Boiling points : Their boiling points are lower than the corresponding acids because of the absence of hydrogen bonding. i.e., ethyl acetate \[={{77.5}^{o}}C\].
(3) Chemical properties
(i) Hydrolysis :
\[\underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+{{H}_{2}}O\,\,\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\,\,+\,\,\underset{\text{Ethyl alcohol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\]
\[\underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+NaOH\xrightarrow{{}}\underset{\text{Sod}\text{. acetate}}{\mathop{C{{H}_{3}}COONa}}\,\,\,+\,\,\underset{\text{Ethyl alcohol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\]
Hydrolysis of ester by alkalies (NaOH) is known as saponification and leads to the formation of soaps
(ii) Reaction with ammonia (ammonolysis) :
![]()
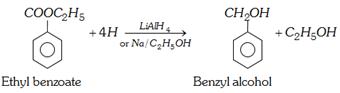
(iii) Reduction
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+4[H]\underset{\text{or }Na/{{C}_{2}}{{H}_{5}}OH}{\mathop{\xrightarrow{LiAl{{H}_{4}}}}}\,2{{C}_{2}}{{H}_{5}}OH\]
\[R-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O{R}'+2{{H}_{2}}\underset{525K,\,200-300\text{atm}}{\mathop{\xrightarrow{CuO.CuC{{r}_{2}}{{O}_{4}}}}}\,RC{{H}_{2}}OH+{R}'OH\]
(iv) Reaction with \[PC{{l}_{5}}\] or \[\mathbf{SOC}{{\mathbf{l}}_{\mathbf{2}}}\]
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+PC{{l}_{5}}\to C{{H}_{3}}COCl+{{C}_{2}}{{H}_{5}}Cl+POC{{l}_{3}}\]
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+SOC{{l}_{2}}\to \underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}COCl}}\,+\underset{\text{Ethyl chloride}}{\mathop{{{C}_{2}}{{H}_{5}}Cl}}\,+S{{O}_{2}}\]
\[\underset{\text{Ethyl benzoate}}{\mathop{{{C}_{\text{6}}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}}}\,+PC{{l}_{5}}\to \underset{\text{Benzoyl chloride}}{\mathop{{{C}_{6}}{{H}_{5}}COCl}}\,+POC{{l}_{3}}+{{C}_{2}}{{H}_{5}}Cl\]
(v) Reaction with alcohols : On refluxing ester undergoes exchange of alcohols residues.
![]()
\[\underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+C{{H}_{3}}OH\to \underset{\text{Methyl acetate}}{\mathop{C{{H}_{3}}COOC{{H}_{3}}}}\,+{{C}_{2}}{{H}_{5}}OH\]
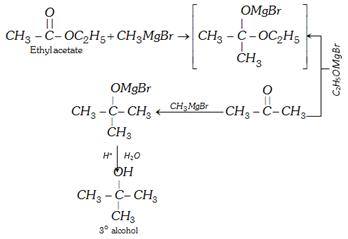
(vi) Reaction with Grignard reagents
(vii) Claisen condensation
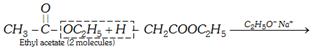
\[\underset{\begin{smallmatrix} \text{Ethyl acetoacetate}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\ \text{(}\beta \text{-ketoester)} \end{smallmatrix}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}}}\,+{{C}_{2}}{{H}_{5}}OH\]
(viii) Reaction with hydroxyl amine

(ix) Reaction with hydrazine
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+\underset{\text{Hydrazine}}{\mathop{{{H}_{2}}NN{{H}_{2}}}}\,\to \underset{\text{Acid hydrazide}}{\mathop{C{{H}_{3}}CONHN{{H}_{2}}}}\,+{{C}_{2}}{{H}_{5}}OH\]
(x) Halogenation
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+B{{r}_{2}}\xrightarrow{\text{Red P}}\underset{\alpha -\text{Bromoethyl acetate}}{\mathop{C{{H}_{2}}BrCOO{{C}_{2}}{{H}_{5}}}}\,+HBr\]
(xi) Reaction with HI
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+HI\to \underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,+\underset{\text{Ethyl alcohol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\]
(4) Uses
(i) As a solvent for oils, fats, cellulose, resins etc.
(ii) In making artificial flavours and essences.
(iii) In the preparation of ethyl acetoacetate.
(5) General Tests
(i) It has sweet smell
(ii) It is neutral towards litmus
(iii) A pink colour is developed when one or two drops of phenolphthalein are added to dilute sodium hydroxide solution. The pink colour is discharged when shaken or warmed with ethyl acetate.
(iv) Ethyl acetate on hydrolysis with caustic soda solution forms two compounds, sodium acetate and ethyl alcohol.
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+NaOH\to C{{H}_{3}}COONa+{{C}_{2}}{{H}_{5}}OH\]
Acid Anhydride
![]()
(1) Method of preparation
(i) From carboxylic acid
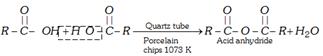
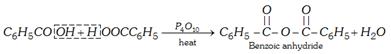
(ii) From carboxylic acid salt and acyl chloride [Laboratory method]
\[C{{H}_{3}}COONa+C{{H}_{3}}COCl\xrightarrow{Py}\underset{\text{Acetic anhydride}}{\mathop{C{{H}_{3}}COOCOC{{H}_{3}}}}\,+NaCl\]
\[{{C}_{6}}{{H}_{5}}COONa+{{C}_{6}}{{H}_{5}}COCl\xrightarrow{Py}\underset{\text{Benzoic anhydride}}{\mathop{{{C}_{6}}{{H}_{5}}COOCO{{C}_{6}}{{H}_{5}}}}\,\]\[+\ NaCl\]
(iii) From acetylene
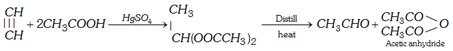
(iv) From acetaldehyde :
\[C{{H}_{3}}CHO+{{O}_{2}}\underset{\text{acetate}}{\mathop{\xrightarrow{\text{Cobalt}}}}\,2C{{H}_{3}}-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-O-O-H\]\[\to {{(C{{H}_{3}}CO)}_{2}}O+{{H}_{2}}O\]
(2) Physical properties
(i) Physical state : Lower aliphatic anhydrides are colourless liquids with sharp irritating smell. The higher members of the family as well as the aromatic acid anhydrides are solids in nature.
(ii) Solubility : They are generally insoluble in water but are soluble in the organic solvents such as ether, acetone, alcohol, etc.
(iii) Boiling points : The boiling points of acid anhydrides are higher than those of carboxylic acids because of the greater molecular size.
(3) Chemical Properties
(i) Hydrolysis :
\[\underset{\text{Acetic anhydride}}{\mathop{C{{H}_{3}}-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-O-\overset{O}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{3}}}}\,+{{H}_{2}}O\to \underset{\text{Acetic acid}}{\mathop{2C{{H}_{3}}COOH}}\,\]
(ii) Action with ammonia
\[{{(C{{H}_{3}}CO)}_{2}}O+2N{{H}_{3}}\to \underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+\underset{\text{Amm}\text{. acetate}}{\mathop{C{{H}_{3}}COON{{H}_{4}}}}\,\]
(iii) Acetylation : Acetic anhydride react with compound having active hydrogen.
\[{{(C{{H}_{3}}CO)}_{2}}O+\underset{\text{Ethyl alcohol}}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\to \underset{\text{Ethyl acetate}}{\mathop{C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}}}\,+C{{H}_{3}}COOH\]
\[{{(C{{H}_{3}}CO)}_{2}}O+\underset{\text{Ethyl amine}}{\mathop{{{H}_{2}}N{{C}_{2}}{{H}_{5}}}}\,\to \underset{N-\text{Ethyl acetamide}}{\mathop{C{{H}_{3}}CONH{{C}_{2}}{{H}_{5}}}}\,+C{{H}_{3}}COOH\]
\[{{(C{{H}_{3}}CO)}_{2}}O+\underset{\text{Diethylamine}}{\mathop{HN{{({{C}_{2}}{{H}_{5}})}_{2}}}}\,\to \underset{N,\,N-\text{Diethyl acetamide}}{\mathop{C{{H}_{3}}CON{{({{C}_{2}}{{H}_{5}})}_{2}}}}\,+C{{H}_{3}}COOH\]
\[{{(C{{H}_{3}}CO)}_{2}}O+\underset{\text{Aniline}}{\mathop{{{H}_{2}}N{{C}_{6}}{{H}_{5}}}}\,\to \underset{\text{Acetanilide}}{\mathop{C{{H}_{3}}CONH{{C}_{6}}{{H}_{5}}}}\,+C{{H}_{3}}COOH\]
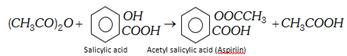
(iv) Action of dry HCl
\[{{(C{{H}_{3}}CO)}_{2}}O+HCl\to C{{H}_{3}}COCl+C{{H}_{3}}COOH\]
(v) Reaction with chlorine
\[{{(C{{H}_{3}}CO)}_{2}}O+C{{l}_{2}}\to \underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}COCl}}\,\,\,+\,\,\underset{\begin{smallmatrix} \text{Monochloroacetic} \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\text{acid} \end{smallmatrix}}{\mathop{C{{H}_{2}}ClCOOH}}\,\]
(vi) Reaction with \[\mathbf{PC}{{\mathbf{l}}_{\mathbf{2}}}\]
\[{{(C{{H}_{3}}CO)}_{2}}O+PC{{l}_{5}}\to 2C{{H}_{3}}COCl+POC{{l}_{3}}\]
(vii) Friedel craft's reaction
\[{{(C{{H}_{3}}CO)}_{2}}O+\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,\xrightarrow{AlC{{l}_{3}}}\underset{\text{Acetophenone}}{\mathop{{{C}_{6}}{{H}_{5}}COC{{H}_{3}}}}\,+C{{H}_{3}}COOH\]
(viii) Reaction with acetaldehyde
\[{{(C{{H}_{3}}CO)}_{2}}O+\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\to \underset{\text{Ethylidene acetate}}{\mathop{C{{H}_{3}}CH{{(OOCC{{H}_{3}})}_{2}}}}\,\]
(ix) Reduction
\[{{(C{{H}_{3}}CO)}_{2}}O\underset{\text{Ether}}{\mathop{\xrightarrow{LiAl{{H}_{4}}}}}\,\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\]
(x) Action with ether :
![]()
(xi) Action with \[{{\mathbf{N}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{5}}}\]
![]()
(4) Uses : Acetic anhydride is used
(i) as an acetylating agent.
(ii) For the detection and estimation of hydroxyl and amino group.
(iii) in the manufacture of cellulose acetate, aspirin, phenacetin, acetamide, acetophenone, etc.
Urea or Carbamide
![]()
Urea may be considered as diamide of an unstable and dibasic carbonic acid from which both the hydroxyl groups have been replaced by \[-N{{H}_{2}}\] groups.
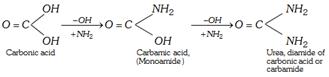
First time isolated from urine in 1773 by Roulle and hence the name urea was given.
(1) Method of preparation
(i) From urine : Urine is treated with conc. nitric acid where crystals of urea nitrate \[CO{{(N{{H}_{2}})}_{2}}.HN{{O}_{3}}\] are obtained.
\[\underset{\text{Urea nitrate}}{\mathop{2CO{{(N{{H}_{2}})}_{2}}.HN{{O}_{3}}}}\,+BaC{{O}_{3}}\to \underset{\text{Urea}}{\mathop{2CO{{(N{{H}_{2}})}_{2}}}}\,+Ba{{(N{{O}_{3}})}_{2}}+{{H}_{2}}O+C{{O}_{2}}\]
(ii) Laboratory preparation
(a) Wohler synthesis
\[\underset{\text{Potassium cyanate}}{\mathop{2KCNO}}\,+\underset{\text{Ammonium sulphate}}{\mathop{{{(N{{H}_{4}})}_{2}}S{{O}_{4}}}}\,\to \underset{\text{Ammonium cyanate}}{\mathop{2N{{H}_{4}}CNO}}\,+{{K}_{2}}S{{O}_{4}}\]
\[\underset{\text{Ammonium cyanate}}{\mathop{N{{H}_{4}}CNO}}\,\underset{\text{On heating}}{\mathop{\xrightarrow{\text{Isomeric change}}}}\,\underset{\text{Urea}}{\mathop{N{{H}_{2}}CON{{H}_{2}}}}\,\]
(b) From phosgene or alkyl carbonate
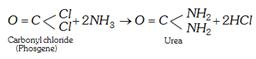
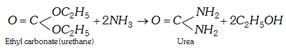
(iii) Industrial method
(a) By partial hydrolysis of calcium cyanide
\[\underset{\begin{smallmatrix} \text{Calcium} \\ \text{Carbide} \end{smallmatrix}}{\mathop{Ca{{C}_{2}}}}\,+{{N}_{2}}\xrightarrow{\text{heat}}\underset{\begin{smallmatrix} \text{Calcium } \\ \text{cyanamide} \end{smallmatrix}}{\mathop{CaC{{N}_{2}}}}\,+C\]
The cyanamide is treated with dilute sulphuric acid at \[{{40}^{o}}C\] where partial hydrolysis occurs with the formation of urea.
\[CaC{{N}_{2}}\underset{-CaS{{O}_{4}}}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,\underset{\text{Cyanamide}}{\mathop{{{H}_{2}}NCN}}\,\underset{({{H}_{2}}{{O}_{2}})}{\mathop{\xrightarrow{{{H}_{2}}O}}}\,\underset{\text{(Urea)}}{\mathop{{{H}_{2}}NCON{{H}_{2}}}}\,\]
or \[CaC{{N}_{2}}+{{H}_{2}}O+{{H}_{2}}S{{O}_{4}}\xrightarrow{{{40}^{o}}C}N{{H}_{2}}CON{{H}_{2}}+CaS{{O}_{4}}\]
(b) From carbon dioxide and ammonia
\[C{{O}_{2}}+2N{{H}_{3}}\xrightarrow{150-{{200}^{o}}C}\underset{\text{Ammonium carbamate}}{\mathop{N{{H}_{2}}COON{{H}_{4}}}}\,\]\[\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{\text{heat}\,\text{(14}{{\text{0}}^{\text{o}}}\text{C)}}}}\,\underset{\text{Urea}}{\mathop{N{{H}_{2}}CON{{H}_{2}}}}\,\]
(2) Physical properties : Urea is a colourless, odourless crystalline solid. It melts at \[{{132}^{o}}C\]. It is very soluble in water, less soluble in alcohol but insoluble in ether, chloroform and benzene.
Crystal structure: In solid urea, both nitrogen atoms are identical.
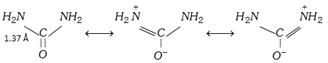
This indicates that \[C-N\] bond in urea has some double bond character.
(3) Chemical Properties
(i) Basic nature (Salt formation): It behaves as a weak monoacid base \[({{K}_{b}}=1.5\times {{10}^{-14}})\]. It forms solt with strong acid.
\[N{{H}_{2}}CON{{H}_{2}}+HN{{O}_{3}}(\text{conc}\text{.})\to \underset{\text{Urea nitrate}}{\mathop{N{{H}_{2}}CON{{H}_{2}}.HN{{O}_{3}}}}\,\]
\[2N{{H}_{2}}CON{{H}_{2}}+\underset{\text{Oxalic acid}}{\mathop{{{H}_{2}}{{C}_{2}}{{O}_{4}}}}\,\to (\underset{\text{Urea oxalate}}{\mathop{N{{H}_{2}}CON{{H}_{2}}{{)}_{2}}{{H}_{2}}{{C}_{2}}{{O}_{4}}}}\,\]
Urea is a stronger base than ordinary amide. It is due to the resonance stabilization of cation, the negatively charged oxygen atom is capable of coordination with one proton.
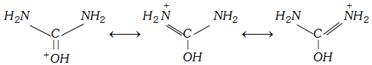
(ii) Hydrolysis
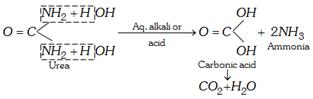
\[N{{H}_{2}}CON{{H}_{2}}+2NaOH\to 2N{{H}_{3}}+N{{a}_{2}}C{{O}_{3}}\]
An enzyme, urease, present in soyabean and soil also brings hydrolysis .
\[N{{H}_{2}}CON{{H}_{2}}+2{{H}_{2}}O\to \underset{\text{Ammonium carbonate}}{\mathop{{{(N{{H}_{4}})}_{2}}C{{O}_{3}}}}\,\to 2N{{H}_{3}}+C{{O}_{2}}+{{H}_{2}}O\]
(iii) Action of heat
![]()
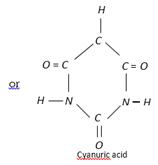
Urea is identified by the test known as biuret test. The biuret residue is dissolved in water and made alkaline with a few drops of NaOH. When a drop of copper sulphate solution is added to the alkaline solution of biuret, a violet colouration is produced.
when heated rapidly at \[{{170}^{o}}C\], polymerisation takes place:
\[N{{H}_{2}}CON{{H}_{2}}\xrightarrow{\text{heat}}N{{H}_{3}}+\underset{\text{Cyanic acid}}{\mathop{HOCN}}\,(H-N=C=O)\]
\[3HOCN\xrightarrow{\text{Polymerisation}}{{(HOCN)}_{3}}\text{or }({{H}_{3}}{{N}_{3}}{{C}_{3}}{{O}_{3}})\]
(iv) Reaction with nitrous acid
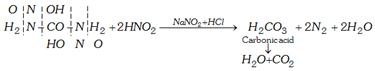
(v) Reaction with alkaline hypohalides
\[NaOH+B{{r}_{2}}\to NaOBr+HBr\]
\[N{{H}_{2}}CON{{H}_{2}}+3NaBrO\to {{N}_{2}}+2{{H}_{2}}O+C{{O}_{2}}+3NaBr\]
(vi) Reaction with acetyl chloride or acetic anhydrides
\[N{{H}_{2}}CON{{H}_{2}}+\underset{\text{Acetyl chloride}}{\mathop{C{{H}_{3}}COCl}}\,\to \underset{\text{Acetyl urea (Ureide)}}{\mathop{N{{H}_{2}}CONHCOC{{H}_{3}}}}\,+HCl\]
\[N{{H}_{2}}CON{{H}_{2}}+{{(C{{H}_{3}}CO)}_{2}}O\to \underset{\text{Acetyl urea}}{\mathop{N{{H}_{2}}CONHCOC{{H}_{3}}}}\,\]\[+\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\]
(vii) Reaction with hydrazine
\[\underset{\text{Urea}}{\mathop{N{{H}_{2}}CON{{H}_{2}}}}\,+\underset{\text{Hydrazine}}{\mathop{{{H}_{2}}N.N{{H}_{2}}}}\,\xrightarrow{{{100}^{o}}C}\underset{\text{Semicarbazide}}{\mathop{N{{H}_{2}}CONH.N{{H}_{2}}}}\,+N{{H}_{3}}\]
(viii) Reaction with ethanol
![]()
(ix) Reaction with chlorine water
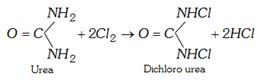
(x) Dehydration
\[N{{H}_{2}}CON{{H}_{2}}+SOC{{l}_{2}}\to {{H}_{2}}N-C\equiv N+S{{O}_{2}}+2HCl+{{H}_{2}}O\]
(xi) Reaction with fuming sulphuric acid
![]()
(xii) Formation of cyclic ureides
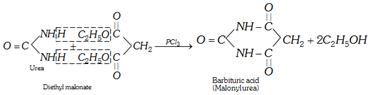
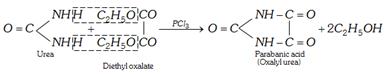
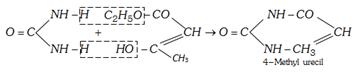
(xiii) Reaction with formaldehyde
\[\underset{\text{Formaldehyde}}{\mathop{C{{H}_{2}}=O+N{{H}_{2}}CON{{H}_{2}}}}\,\xrightarrow{HCl}\underset{\text{Monomethylol urea}}{\mathop{C{{H}_{2}}(OH)NHCON{{H}_{2}}}}\,\xrightarrow{C{{H}_{2}}=O}\]
\[\underset{\text{Dimethylol urea}}{\mathop{C{{H}_{2}}(OH)NHCONH(OH)C{{H}_{2}}}}\,\,\,\,\,\,\,\,\xrightarrow{heat}\,\,\underset{\text{(Urea-Formaldehyde)}}{\mathop{\operatorname{Re}\sin }}\,\]
(4) Uses
(i) Mainly as a nitrogen fertilizer. It has 46.4% nitrogen.
(ii) In the manufacture of formaldehyde-urea plastic and semicarbazide.
(iii) As animal feed.
(iv) For making barbiturates and other drugs.
(v) As a stabilizer for nitrocellulose explosives.
(5) General Tests
(i) When heated with sodium hydroxide, ammonia is evolved.
(ii) When heated gently, it forms biuret which gives violet colouration with sodium hydroxide and a drop of copper sulphate solution.
(iii) Its aqueous solution with concentrated nitric acid gives a white precipitate.
(iv) On adding sodium nitrite solution and dil. \[HCl\] (i.e., \[HN{{O}_{2}}\]) to urea solution, nitrogen gas is evolved and gives effervescence due to carbon dioxide.
You need to login to perform this action.
You will be redirected in
3 sec
