Glucose; \[({{C}_{6}}{{H}_{12}}{{O}_{6}})\] or Aldo-hexose
Category : JEE Main & Advanced
Glucose is known as dextrose because it occurs in nature as the optically active dextrorotatory isomer. It is also called grape sugar as it is found in most sweet fruits especially grapes.
(1) Preparation :
(i) Laboratory method
\[\underset{\text{(Sucrose)}}{\mathop{\underset{\text{Cane sugar}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,}}\,+{{H}_{2}}O\xrightarrow{{{H}^{+}}}\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
HCl (dil.) is used for hydrolysis. Glucose being much less soluble in alcohol than fructose separates out by crystallising on cooling. (ii) Manufacture : It is obtained on a large scale by the hydrolysis of starch (corn starch or potato starch) with dilute sulphuric acid or hydrochloric acid. \[\underset{\text{Starch}}{\mathop{{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}}}\,+n{{H}_{2}}O\underset{320{}^\circ C,\,2-3\,atm}{\mathop{\xrightarrow{{{H}^{+}}}}}\,\underset{\text{Glucose}}{\mathop{n{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
A thin paste of starch is boiled with dilute acid till the hydrolysis is complete. The excess of acid is neutralised with chalk (calcium carbonate) and the filtrate containing glucose is decolourised with animal charcoal. The solution is concentrated and evaporated under reduced pressure. Glucose is obtained in crystalline form.
(2) Physical properties : It is a colourless crystalline solid, melts at \[{{146}^{o}}C\]. It is readily soluble in water. From aqueous solution, it separates as a crystalline monohydrate \[({{C}_{6}}{{H}_{12}}{{O}_{6}}.{{H}_{2}}O)\] which melts at \[{{86}^{o}}C\]. It is sparingly soluble in alcohol but insoluble in ether. It is less sweet (three-fourth) than cane sugar. It is optically active and the ordinary naturally occuring form is (+) glucose or dextro form. It shows mutarotation.
(3) Chemical properties
(i) Alcoholic reactions (Reactions due to -OH group) :
(a) Reaction with acid chlorides and acid anhydride
\[\underset{\text{Glucose}}{\mathop{\begin{matrix} CHO\ \ \ \ \\ |\ \ \ \ \ \ \ \ \ \ \\ {{(CHOH)}_{4}} \\ |\ \ \ \ \ \ \ \ \ \ \\ C{{H}_{2}}OH \\ \end{matrix}}}\,+\underset{\text{Acetyl chloride}}{\mathop{5C{{H}_{3}}COCl}}\,\xrightarrow{ZnC{{l}_{2}}}\underset{\text{Glucose penta-acetate}}{\mathop{\begin{matrix} CHO\ \ \ \ \,\,\,\,\,\,\,\,\,\,\,\,\, \\ |\ \ \ \ \ \ \ \,\,\,\,\,\,\,\,\,\,\,\,\,\ \ \ \\ {{(CHOOCC{{H}_{3}})}_{4}} \\ |\ \ \,\,\,\,\,\,\,\,\,\,\,\,\,\ \ \ \ \ \ \ \ \\ C{{H}_{2}}OOCC{{H}_{3}} \\ \end{matrix}}}\,+5HCl\]
This shows that a molecule of glucose contains 5 - OH groups.
(b) Reaction with \[PC{{l}_{5}}\]
\[\underset{\text{Glucose}}{\mathop{\begin{matrix} CHO\,\,\,\,\,\, \\ |\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\ {{(CHOH)}_{4}} \\ |\,\,\,\,\,\,\,\,\,\,\,\,\,\,\, \\ C{{H}_{2}}OH \\ \end{matrix}}}\,+5PC{{l}_{5}}\underset{\text{(Glucose penta-chloride)}}{\mathop{\underset{\,\text{Penta-chloroglucose}}{\mathop{\xrightarrow{{}}\,\,\begin{matrix} CHO\,\,\,\, \\ |\,\,\,\,\,\,\,\,\,\,\,\,\, \\ {{(CHCl)}_{4}} \\ |\,\,\,\,\,\,\,\,\,\,\,\,\, \\ C{{H}_{2}}Cl \\ \end{matrix}}}\,}}\,+5POC{{l}_{3}}+5HCl\]
(c) Reaction with metallic hydroxides
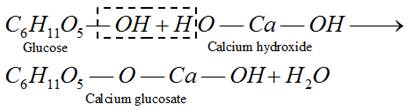
(d) Formation of glycosides
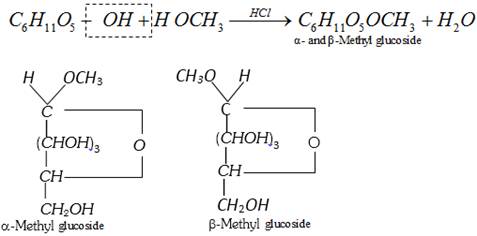
This reaction shows the presence of ring structure in glucose.
(ii) Reactions of carbonyl group (Aldehydic group)
(a) Reduction
\[\underset{\text{Glucose}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CHO}}\,+2H\underset{{{H}_{2}}O}{\mathop{\xrightarrow{Na-Hg}}}\,\underset{\text{Sorbitol}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}C{{H}_{2}}OH}}\,\] On prolonged heating with concentrated HI and red phosphorus at \[{{110}^{o}}C\], glucose forms a mixture of 2-iodohexane and n-hexane.
(b) Oxidation
\[\underset{\text{Glucose}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CHO}}\,+2CuO\xrightarrow{{}}\] \[\underset{\text{Gluconic acid}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}COOH}}\,\underset{\text{(red ppt}\text{.)}}{\mathop{+C{{u}_{2}}O}}\,\].
\[C{{H}_{2}}OH{{(CHOH)}_{4}}CHO+A{{g}_{2}}O\xrightarrow{{}}\] \[C{{H}_{2}}OH{{(CHOH)}_{4}}COOH+\underset{\text{ }\!\![\!\!\text{ or black ppt}\text{. }\!\!]\!\!\text{ }}{\mathop{\underset{\text{(Mirror)}}{\mathop{2Ag}}\,}}\,\].
\[\underset{\text{Glucose}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CHO}}\,+[O]\xrightarrow{B{{r}_{2}}/{{H}_{2}}O}\] \[\underset{\text{Gluconic acid}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}COOH}}\,\].
\[\underset{\text{Glucose (}{{C}_{\text{6}}})}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CHO}}\,+3[O]\xrightarrow{HN{{O}_{3}}}\] \[\underset{\text{Saccharic acid (}{{C}_{\text{6}}}\text{)}}{\mathop{COOH{{(CHOH)}_{4}}COOH}}\,+{{H}_{2}}O\].
(c) Reaction with HCN
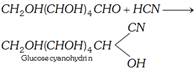
(d) Reaction with hydroxyl amine
\[C{{H}_{2}}OH{{(CHOH)}_{4}}CHO+N{{H}_{2}}OH\to \] \[\underset{\text{Glucose oxime}}{\mathop{C{{H}_{2}}OH{{(CHOH)}_{4}}CH}}\,=NOH+{{H}_{2}}O\]
(e) Reaction with Phenyl hydrazine (Fischer's mechanism) : When warmed with excess of phenyl hydrazine, glucose first forms phenylhydrazone by condensation with -CHO group.
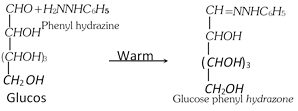
The adjacent -CHOH group is then oxidised by a second molecule of phenyl hydrazine.
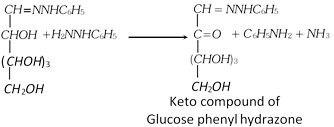
The resulting carbonyl compounds reacts with a third molecule of phenyl hydrazine to yield glucosazone.
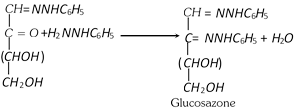
(iii) Miscellaneous reactions
(a) Fermentation \[\underset{\text{Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\xrightarrow{Zymase}\underset{\text{Ethanol}}{\mathop{2{{C}_{2}}{{H}_{5}}OH}}\,+2C{{O}_{2}}\]
(b) Dehydration : When heated strongly or when treated with warm concentrated sulphuric acid, glucose is dehydrated to give a black mass (sugar carbon).
(c) Reaction with alkalies : When warmed with concentrated alkali, glucose first turns yellow; then brown and finally gives a resinous mass.
A dilute solution of glucose, when warmed with dilute solution of alkali, some glucose is converted into fructose and mannose. D-glucose and D-mannose are epimers.
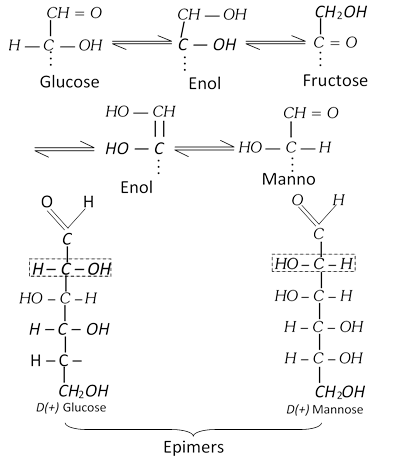
(d) Action of concentrated hydrochloric acid
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\xrightarrow{Conc.\,HCl}\] \[\underset{\text{Laevulic acid}}{\mathop{C{{H}_{3}}COC{{H}_{2}}C{{H}_{2}}COOH}}\,+HCOOH+{{H}_{2}}O\]
On treatment with conc. \[HCl,\] glucose can also form hydroxymethyl furfural.
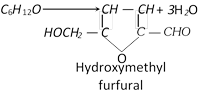
This on acid treatment gives laevulic acid
(4) Uses
(i) In the preservation of fruits and preparation of jams and jellies.
(ii) In the preparation of confectionary and as a sweetening agent.
(iii) As a food for patients, invalids and children.
(iv) In the form of calcium glucosate as medicine in treatment of calcium deficiency.
(v) As a reducing agent in silvering of mirrors.
(vi) As a raw material for alcoholic preparations.
(vii) In industrial preparation of vitamin-C.
(viii) In the processing of tobacco.
(ix) As an intravenous injection to the patients with lower glucose content in blood.
(5) Test of glucose
(i) When heated in a dry test tube, it melts, turns brown and finally black, giving a characteristic smell of burnt sugar.
(ii) When warmed with a little conc. \[{{H}_{2}}S{{O}_{4}}\], it leaves a charred residue of carbon.
(iii) When it is boiled with dilute \[NaOH\] solution, it first turns yellow and then brown.
(iv) Molisch's test : This is a general test for carbohydrates. Two or three drops of alcoholic solution of a-naphthol is added to 2mL of glucose solution. 1 mL of concentrated \[{{H}_{2}}S{{O}_{4}}\] is added carefully along the sides of the test tube. The formation of a violet ring, at the junction of two liquids confirms the presence of a carbohydrate.
(v) Silver mirror test : A mixture of glucose and ammonical silver nitrate is warmed in a test tube. Appearance of silver mirror on the inner walls confirms glucose.
(vi) Fehling's solution test : A little glucose is warmed with Fehling?s solution. A red precipitate of cuprous oxide is formed.
(vii) Osazone formation : Glucose on heating with excess of phenyl hydrazine in acetic acid gives a yellow crystalline compound, m.pt. \[{{205}^{o}}C\].
(6) Structure of glucose
(i) Open chain structure : The structure of D-glucose as elucidated by Emil Fischer is,
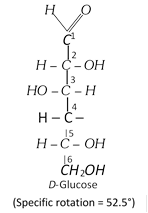
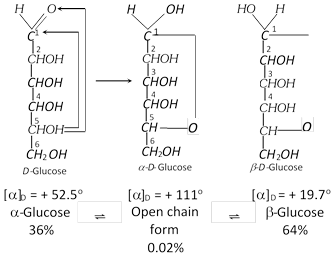
(b) Ordinary glucose is a-glucose, with a fresh aqueous solution has specific rotation, \[{{[\alpha ]}_{D}}+{{111}^{o}}.\] On keeping the solution for some time; a-glucose slowly changes into an equilibrium mixture of a-glucose (36%) and b-glucose (64%) and the mixture has specific rotation + 52.5o.
Similarly a fresh aqueous solution of b-glucose having specific rotation, \[{{[\alpha ]}_{D}}+{{19.7}^{o}}\], on keeping (standing) gradually changes into the same equilibrium mixutre (having, specific rotation \[+{{52.7}^{o}}).\] So an aqueous solution of glucose shows a physical property, known as mutarotation, i.e., a change in the value of specific rotation (muta=change; rotation = specific rotation) is called mutarotation.
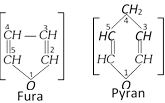
(c) Fischer and Tollen's proposed that the ring or the internal hemiacetal is formed between \[{{C}^{1}}\] and \[{{C}^{4}}\]. It means the ring is Furan type or 5-membered ring; this is called Furanose strucutre,
However according to Haworth and Hirst the ring is formed between \[{{C}^{1}}\] and \[{{C}^{5}}\]. It means the ring is Pyran type or 6-membered ring, this is called Pyranose structure.
(d) Haworth structure : The two forms of D-glucose are also shown by Haworth projection formula which are given below, 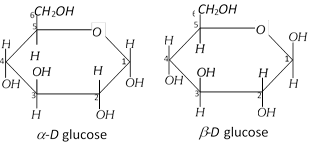
You need to login to perform this action.
You will be redirected in
3 sec
