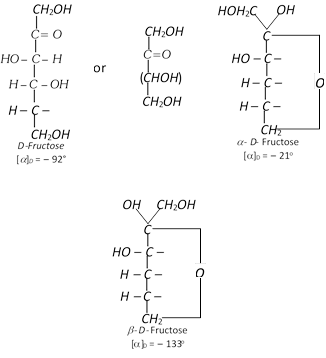
Fructose, fruit sugar \[({{C}_{6}}{{H}_{12}}{{O}_{6}})\] or ketohexose
Category : JEE Main & Advanced
It is present in abundance in fruits and hence is called fruit sugar. It is also present in cane sugar and honey alongwith glucose in combined form. The polysaccharide inulin is a polymer of fructose an gives only fructose on hydrolysis. Since naturally occurring fructose is laevorotatory, it is also known as laevulose.
(1) Preparation :
(i) Hydrolysis of cane sugar
\[\underset{\text{Cane sugar}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+{{H}_{2}}O\underset{\text{Warm}}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}\text{(dil}\text{.)}}}}\,\underset{\text{D-Glucose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{D-Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
The solution having equal molecules of D-glucose and D-fructose is termed invert sugar and the process is known as inversion.
\[\underset{\text{Calcium fructose}}{\mathop{{{C}_{6}}{{H}_{11}}{{O}_{5}}-O-CaOH}}\,+C{{O}_{2}}\xrightarrow{{}}\underset{\text{Fructose}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+CaC{{O}_{3}}\]
(ii) Hydrolysis of Inulin with dilute sulphuric acid
\[\underset{\text{Inulin}}{\mathop{{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}}}\,+n{{H}_{2}}O\xrightarrow{{{H}_{2}}S{{O}_{4}}\text{(dil}\text{.)}}\underset{\text{Fructose}}{\mathop{n{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
(2) Properties : The anhydrous fructose is a colourless crystalline compounds. It melts at \[{{102}^{o}}C.\] It is soluble in water but insoluble in benzene and ether. It is less soluble in water than glucose. It is the sweetest of all sugars and its solution is laevorotatory. Like glucose, it also shows mutarotation.
Comparison between glucose and fructose
| Property | Glucose | Fructose |
| Molecular formula | \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\] | \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\] |
| Nature | Polyhydroxy aldehyde. | Polyhydroxy ketone |
| Melting point | \[146{}^\circ C\] | \[102{}^\circ C\] |
| Optical activity of natural form | Dextrorotatory | Laevorotatory |
| With ethyl alcohol | Almost insoluble | More soluble |
| Oxidation (a) With bromine water (b) With nitric acid | Gluconic acid Saccharic acid (Glucaric acid) | No reaction Mixture of glycollic acid, tartaric acid and trihydroxy glutaric acid |
| Reduction | Sorbitol | Mixture of sorbitol and mannitol |
| Calcium hydroxide | Forms calcium glucosate, soluble in water | Forms calcium fructosate, insoluble in water |
| Molisch's reagent | Forms a violet ring | Forms a violet ring |
| Fehling's solution | Gives red precipitate | Gives red precipitate |
| Tollen's reagent | Forms silver mirror | Forms silver mirror |
| Phenyl hydrazine | Forms osazone | Forms osazone |
| Resorcinol + HCl (dil.) (Selivanoff's test) | No colouration | Gives red or brown colour or precipitate |
| Freshly prepared ammonium molybdate sol. + few drops of acetic acid (Pinoff's test). | Light blue colour | Bluish green colour on heating |
| Alcoholic a-naphthol + HCl (conc.) (Furfural test) | No colouration | A purple colour (violet) on boiling |
Interconversions :
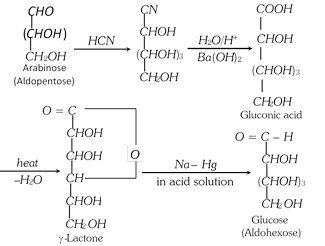
(1) Chain Lengthening of Aldoses (Killiani-Fischer synthesis) : The conversion of an aldose to the next higher member involves the following steps :
(i) Formation of a cyanohydrin.
(ii) Hydrolysis of - CN to -COOH forming aldonic acid.
(iii) Conversion of aldonic acid into lactone by heating.
(iv) The lactone is finally reduced with sodium amalgam or sodium borohydride to give the higher aldose.
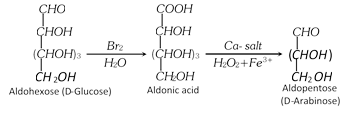
(2) Chain Shortening of aldoses
(i) An aldose can be converted to the next lower member by Ruff Degradation.
It involves two steps.
(ii) By Wohl's method
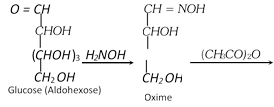
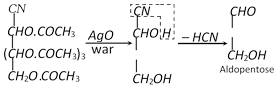
(3) Conversion of an aldose to the isomeric Ketose
Three steps are involved,
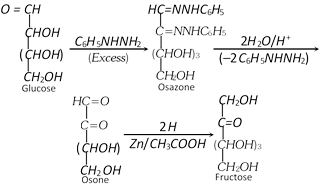
(4) Conversion of a ketose to the isomeric aldose
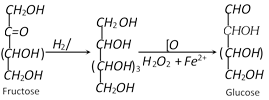
You need to login to perform this action.
You will be redirected in
3 sec
