Derivatives Of Phenol
Category : JEE Main & Advanced
nitrophenols
(1) Preparation
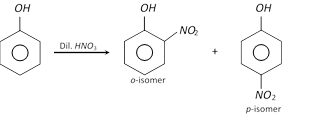
(ii) 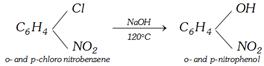
(iii) 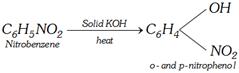
(iv) 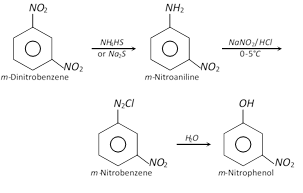
(2) Properties : o-Nitrophenol is a yellow coloured crystalline compound, while m- and p-isomers are colourless crystalline compounds.
\[\begin{matrix} \text{Isomer} \\ \text{m}\text{.pt}\text{. (}{}^\circ \text{C)} \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} ortho \\ 45 \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} meta \\ 97 \\ \end{matrix}\,\,\,\,\,\,\begin{matrix} para \\ 114 \\ \end{matrix}\]
The lowest melting point of o-isomer is due to intramolecular hydrogen bonding whereas meta and para isomers possess intermolecular hydrogen bonding and thus, they have higher melting points.
They are stronger acids than phenol. The order is :
p-isomer > o-isomer > m-isomer > phenol
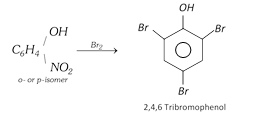
When reduced, they form corresponding aminophenols. o- and p-Nitrophenols react with bromine water to form 2, 4, 6-tribromophenol by replacement of nitro group.
Picric acid (2, 4, 6-trinitrophenol)
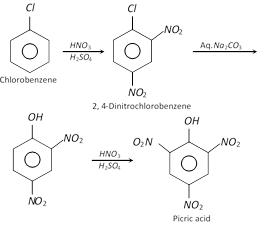
(1) Preparation : It is obtained when phenol is treated with conc. \[HN{{O}_{3}}\]. However, the yield is very poor. It is prepared on an industrial scale :
(i) From chlorobenzene
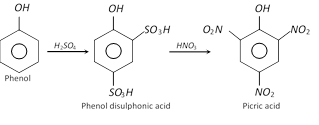
(ii) From phenol through disulphonic acid
(iii)
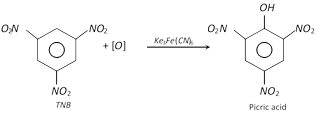
(2) Properties : It is a yellow crystalline solid, melting points \[{{122}^{o}}C\]. it is insoluble in cold water but soluble in hot water and in ether. It is bitter in taste. Due to the presence of three electronegative nitro groups, it is a stronger acid than phenol and its properties are comparable to the carboxylic acid. It neutralises alkalies and decomposes carbonates with evolution of carbon dioxide.
Dry picric acid as well as its potassium or ammonium salts explode violently when detonated. It reacts with \[PC{{l}_{5}}\] to form picryl chloride which on shaking with \[N{{H}_{3}}\] yields picramide.
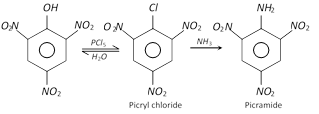
When distilled with a paste of bleaching powder, it gets decomposed and yields chloropicrin, \[CC{{l}_{3}}N{{O}_{2}}\], as one of the products and is thus employed for the manufacture of tear gas.
It forms yellow, orange or red coloured molecular compounds called picrates with aromatic hydrocarbons, amines and phenols which are used for characterisation of these compounds.
(3) Uses : It is used as a yellow dye for silk and wool, as an explosive and as an antiseptic in treatment of burns.
Catechol (1, 2-Dihydroxy benzene)
(1) Preparation
(i) 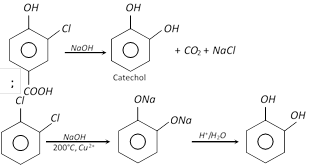
(ii) 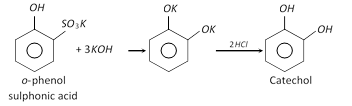
(iii) 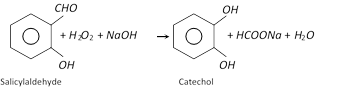
(2) Properties : It is a colourless crystalline solid, melting points \[{{105}^{o}}C\]. it is soluble in water. It is affected on exposure to air and light. It acts as a reducing agent as it reduces Tollen’s reagent in cold and Fehling’s solution on heating. With silver oxide it is oxidised to o-benzoquinone.
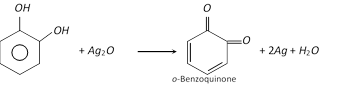
It forms insoluble lead salt (white ppt.) when treated with lead acetate solution and gives green colour with \[FeC{{l}_{3}}\] which changes to red on adding \[N{{a}_{2}}C{{O}_{3}}\] solution. It forms alizarin dye stuff when condensed with phthalic anhydride in the presence of sulphuric acid.
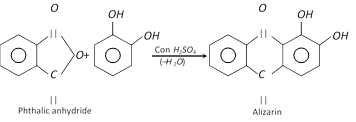
(3) Uses : It finds use as photographic developer, in the manufacture of alizarin and adrenaline hormone and as an antioxidant (inhibitor in auto oxidation) for preserving gasoline.
Resorcinol (1, 3-Dihydroxy benzene)
(1) Preparation : It is prepared by alkali fusion of 1,3, benzene disulphonic acid (Industrial method).
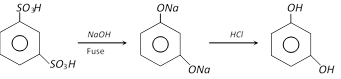
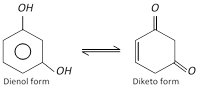
(2) Properties : It is a colourless crystalline solid, melting points \[{{110}^{o}}C\]. it is affected on exposure by air and light. It is soluble in water, alcohol and ether. It shows tautomerism. Its aqueous solution gives violet colour with \[FeC{{l}_{3}}\]. It reduces Fehling’s solution and Tollen’s reagent on warming.
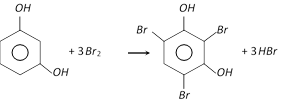
With bromine water, it gives a crystalline precipitate, 2, 4, 6-tribromoresorcinol.
On nitration, it forms 2, 4, 6-trinitro-1, 3-dihydroxybenzene.
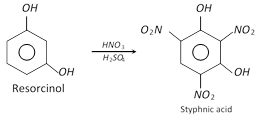
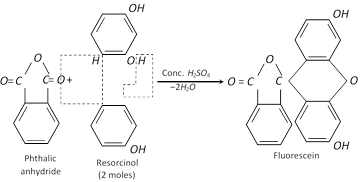
It condenses with phthalic anhydride and forms fluorescein.
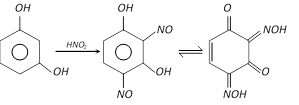
With nitrous acid, it forms 2, 4-dinitrosoresorcinol
Resorcinol behaves as a tautomeric compound. This is shown by the fact that it forms a dioxime and a bisulphite derivative.
(3) Uses
(i) It is used as antiseptic and for making dyes.
(ii) It is also used in the treatment of eczema. 2, 4, 6-trinitroresorcinol is used as an explosive.
Hydroquinone or quinol (1, 4-Dihydroxy benzene)
(1) Preparation : It is formed by reduction of p-benzoquinone with sulphurous acid \[({{H}_{2}}S{{O}_{3}}={{H}_{2}}O+S{{O}_{2}})\].
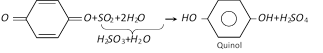
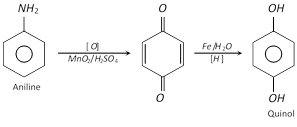
(p-Benzoquinone is obtained by oxidation of aniline)
(2) Properties : It is a colourless crystalline solid, melting points \[{{170}^{o}}C\]. it is soluble in water. It also shows tautomerism. It gives blue colour with \[FeC{{l}_{3}}\] solution.
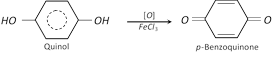
Due to this property, it is used as photographic developer.
(3) Uses : It is used as an antiseptic, developer in photography, in the preparation of quinhydrone electrode and as an antioxidant.
Trihydric Phenols : Three trihydroxy isomeric derivatives of benzene are Pyrogallol (1, 2, 3), hydroxy quinol (1, 2, 4) and phloroglucinol (1, 3, 5).
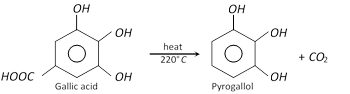
Pyrogallol is obtained by heating aqueous solution of gallic acid at \[{{220}^{o}}C\].
Phloroglucinol is obtained from trinitrotoluene (TNT) by following sequence of reactions.
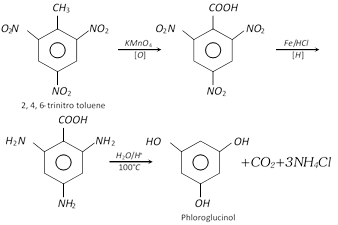
Hydroxyquinol is prepared by the alkaline fusion of hydroquinone in air.
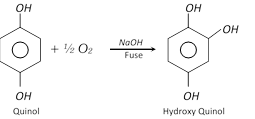
The three isomers are colourless crystalline compounds. All are soluble in water and their aqueous solutions give characteristic colour with \[FeC{{l}_{3}}\] (Red, brown or bluish violet). Alkaline solutions absorb oxygen rapidly from air.
Uses of pyrogallol
(i) As a developer in photography.
(ii) As a hair dye.
(iii) In treatment of skin diseases like eczema.
(iv) For absorbing unreacted oxygen in gas analysis.
You need to login to perform this action.
You will be redirected in
3 sec
