Physical States Matter
Category : 9th Class
Everything in this universe is made up of matter. All the things we see in our surroundings occupies space, and has certain mass and volume. Early Indian philosopher used to says that, matters are classified in the form of five basic elements, called panch tatva. These panch tatva includes air, water, earth, sky and fire. According to them everything whether living or non living is made up of only these five things. But modern classification is based on the physical and chemical properties of the matter. Chemistry deals with the study of matters. It involves many aspects of matters which are based on its classification and on the chemical composition into three main categories namely elements, compounds and metalloids.
![]() Physical States of Matter
Physical States of Matter
For long time it is believed that the matters exist in two forms. First it exists in the form of a block like woods; Secondly, it is made up of particles like sand and dust. But modern aspect believes that the particle of matters are very- small, which are beyond our imagination.
![]() Characteristics of Particles of Matter
Characteristics of Particles of Matter
The different characteristics of particles of matter are:
When we put sugar or salt in water we see that after some time it disappears.
Where does these particles goes? The answer to this question is, actually as there is space between the particles of water molecules, the sugar or the salt molecules get into the space between the molecules of the water. Thus we can say that particles of matters have space between them.
We know that the particle of matters possess kinetic energy. As the temperature increases the kinetic energy increases and hence the particles start moving faster.
Take two beakers and put some hot water in one and cold water in the other.
Put equal mass of sugar in both the beaker and left them undisturbed. After sometime you will observe that the sugar have dissolved faster in the beaker containing hot water. This is because the hot water molecule has higher kinetic energy and hence moves faster to dissolves the sugar molecules in comparision to the cold water.
Spread a glass of water on the plane surface of table, which is slightly sloped in one direction. Take a pin and drag one end of the water towards the slope. You will observe that the water molecules has started flowing in that direction. This shows that particles of matters attract each other.

![]() State of Matter
State of Matter
Matters have three physical states that is solid, liquid, and gas. The different states of matter is due to the variation in characteristics of the particles of matter . We will discuss about all the three states of matter, in detail.
![]() Solid
Solid
Solids are the matter, that have definite shape, certain mass and fixed volume. Its compression is negligible. It has highest density among all the three states. The intermolecular force of attraction is highest. Molecules in the solid are closely packed. They cannot flow because the constituent particles are held closely in fixed position, due to strong force of attraction.

![]() Liquid
Liquid
Liquids are the substances, which do not have any fixed shape, but has fixed mass and volume. It always takes the shape of the container in which it is kept. In liquid, the molecules are loosely packed. The intermolecular force of attraction is less than solid, and density is also less. It can easily flow from one place to another. Particles of liquid have greater space between them. The rate of diffusion of liquid is higher than solid. This is due to the fact that particles of liquid have greater space between them and can move freely as compared to the particles of solid. They are more compressible than solid.

![]() Gas
Gas
Gas is the substance in which molecules are free to move about and have no fixed shape or volume. They can easily flow from one place to another. They are highly compressible. The intermolecular distance is maximum, force is minimum. The density of gas is least as compared to solid or liquid. In gas, the particles move about randomly at high speed. Due to this movement of the particles, they hit each other and also, the wall of the container. Thus, gas exert pressure on the wall of the container, in which it is enclosed. The pressure exerted by the gas on the wall of the container is equal to the force, exerted by the gas particles per unit area, on the wall of the container.


![]() Which one of the following matters exist in all the three states?
Which one of the following matters exist in all the three states?
(a) Petrol
(b) Mercury
(c) Iron
(d) Water
(e) None of these
Answer: (d)
![]() Which one of the following undergoes sublimation?
Which one of the following undergoes sublimation?
(a) Ice
(b) Potassium
(c) Camphor
(d) Water
(e) None of these
Answer: (c)
![]() Which one of following has highest density?
Which one of following has highest density?
(a) Petrol
(b) Water
(c) Stone
(d) Mercury
(e) None of these
Answer: (c)
![]() In which matter the force of cohesion is maximum?
In which matter the force of cohesion is maximum?
(a) Steel
(b) Water
(c) Milk
(d) Oxygen
(e) None of these
Answer: (a)
![]() Which one of the following characteristic is fixed in the liquid?
Which one of the following characteristic is fixed in the liquid?
(a) Shape
(b) Volume
(c) Rigidity
(d) All of these
(e) None of these
Answer: (b)
![]() Which form of the matter is more stable at lower temperature?
Which form of the matter is more stable at lower temperature?
(a) Solid
(b) Liquid
(c) Gas
(d) Plasma
(e) None of these
Answer: (a)
![]() Which one of the following is indefinite in case of liquid?
Which one of the following is indefinite in case of liquid?
(a) Volume
(b) Mass
(c) Shape
(d) Force
(e) None of these
Answer: (c)
![]() Change of the States of Matter
Change of the States of Matter
We all know that matter can change its state from one form to another i.e. from solid to liquid or liquid to gas or gas to solid and vice versa. The big question which arises during such changes is that what happens to the particle inside the matters, during the change of states? How does this changes takes places? When we study and analyses such situation we find that there are many factors responsible for such change and pressure temperature.
![]() Effect of Temperature
Effect of Temperature
If we increase the temperature of the solid, the particles of the solid gains kinetic energy and starts vibrating with greater speed. As the vibration increases, the particles overcome the force of attraction between them and starts moving more freely. If the heating is prolonged, then the solid starts melting. The temperature at which solid starts melting is called the melting point of solid. Different solid have different melting points. The difference in the melting points of the solid is due to the difference in the intermolecular force of attraction, between the molecules of the substance. The conversion of solid into liquid is known as fusion. We observe that when a solid melts its temperature remains the same, even if we keeps on heating. Actually this heat is used in overcoming the force of attraction between the particles of the matter. This heat energy is absorbed by the particles of the matter and remains hidden in the matter, so it is called the latent heat. Thus we can define the latent heat as the amount of heat energy required to change 1 kg of a solid into liquid at its atmospheric pressure without changing the temperature. This is also known as the latent heat of fusion.
The boiling point of water is found to be 100°C = 373 K.
When substance changes from liquid to gas, it is called the evaporation. The temperature at which liquid changes into gas is called latent heat of vaporization. Some time it happens that solid directly have to be changed into gas without changing into liquid or vice versa. This process is called the sublimation. For example, if we heat camphor or ammonium chloride it gets converted into gas and if we condense it again it will get reconverted into solid.
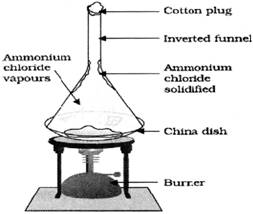
![]() Effect of Pressure
Effect of Pressure
Pressure also plays very important role in interconversion of states of matter. If we compress a gas by applying a pressure in a closed cylinder, we find that it gets converted into liquid. But if we compress a liquid by applying the pressure and reducing temperature it get converted into solid, like freezing of liquid into ice.
For example, we know that solid carbondioxide, also known as dry ice, is always stored at high pressure. If we reduce the pressure, it directly get converted into gas, without changing into liquid. Thus we can say that pressure and temperature determines the states of matter. 1 atmospheric pressure = 1.01 x 105 Pa.
![]() Factors Affects the Rate of Evaporation
Factors Affects the Rate of Evaporation
The various factors that affects the rate of evaporations are:
![]() Condensation
Condensation
The process of conversion of vapour into liquid is called condensation.
![]() Plasma
Plasma
It is the states of matter which consists of super energetic and super excited particles. These particles are in the form of ionized gases. For example, the fluorescent tube and neon bulbs consists of plasma. Inside the fluorescent tube is helium gas and inside neon bulb is neon gas. When we pass electric current through it, the gas gets ionized and charged, which cause the plasma to glow inside the tube.
![]() Vaporation Cause Cooling
Vaporation Cause Cooling
When the liquid evaporates from its surface, it looses energy to overcome the intermolecular force of attraction. The lost energy is gained by the particles of the liquid, which absorbs energy from the surroundings. This absorption of energy from the surroundings makes the surrounding cool. We normally wear cotton clothes during summer. In summer we perspire more. These water vapours absorbs, heat from the surroundings and hence cause cooling. Cotton being good absorber of sweat, absorbs the sweat, leaving the body cool.
Difference between Solid, Liquid and Gas
| S. No. | Solid | Liquid | Gas |
| 1. | It has fixed shape and definite volume. | It do not have any fixed shape/ but have fixed volume. | It has neither fixed shape nor fixed volume. |
| 2. | It has high density. | Its density is less than solid but more than liquid. | It has least density. |
| 3. | It is slightly compressible. | It is more compressible than solid. | It is highly compressible. |
| 4. | It has strongest intermolecular force of attraction. | Intermolecular force of attraction is more than gas and less than solid. | It has weakest intermolecular force of attraction. |
| 5. | It has highest density. | Its density is less than solid. | It has least density among all. |
| 6. | It cannot flow. | It can flow. | It can flow freely. |

![]() In which one of the following cases the intermolecular force is weakest?
In which one of the following cases the intermolecular force is weakest?
(a) Wood
(b) Mercury
(c) Iron
(d) Oxygen
(e) None of these
Answer: (d)
![]() Which among the following is not a matter?
Which among the following is not a matter?
(a) Boiling point
(b) Ice
(c) Wood
(d) Bulb
(e) None of these
Answer: (a)
![]() The state of matter which consist of gases is called:
The state of matter which consist of gases is called:
(a) Solid
(b) Liquid
(c) Blood
(d) Plasma
(e) None of these
Answer: (d)
![]() Helium gas is a matter because:
Helium gas is a matter because:
(a) It has mass and occupies volume
(b) It has no definite volume
(c) It can be compressed easily
(d) It has a mass
(e) None of these
Answer: (a)
![]() Which among the following has strongest intermolecular force?
Which among the following has strongest intermolecular force?
(a) Carbon dioxide
(b) Sodium carbonate
(c) Sodium chloride
(d) Chlorine
(e) None of these
Answer: (c)
![]() Which phenomenon is responsible for spread of perfumes from one corner of the room to the other?
Which phenomenon is responsible for spread of perfumes from one corner of the room to the other?
(a) Fusion
(b) Melting
(c) Evaporation
(d) Diffusion
(e) None of these
Answer: (d)
![]()
You need to login to perform this action.
You will be redirected in
3 sec
