Chemical Equations
Category : 7th Class
Chemical equation is the symbolic representation of a chemical reaction. The reactants of the reaction are represented on the left hand side of the equation and products are represented on right hand side. The representation of reactants in an equation are in symbolic form. Plus sign between the reactants separates every reactant. On the right hand side, every product is also separated by plus sign. The arrow mark (read as yield) between the reactants and resulting elements is used to denote the net forward reaction.
Following symbol is used between the reactants and products.
= Symbol is used to denote a stoichiometric relation.
![]() Symbol is used to denote the chemical reaction in both directions or reversible reaction.
Symbol is used to denote the chemical reaction in both directions or reversible reaction.
![]() Symbol is used to denote equilibrium.
Symbol is used to denote equilibrium.
The physical state of the chemicals are denoted in parenthesis after the symbol of the chemicals. The letter s in parenthesis (s) denotes the solid state of the chemicals. The letter l in parenthesis (I) denotes the liquid state of the chemicals and letter g in parenthesis (g) denotes the gaseous state of the chemicals. The letter aq in parenthesis (aq) denotes the aqueous solutions. The Greek letter delta (a) above the arrow denotes the energy in the form of heat is added to the reaction.
The letter hv above the arrow is used to denote the energy in the form of light is added to the reaction.
Look at the following picture of chemical equation
Reactants Products
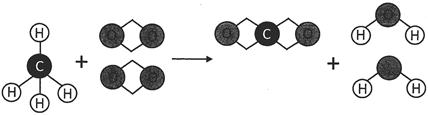
In the above picture, methane and oxygen are reactants and carbon dioxide and water are products. An arrow mark between the reactants and the products is used to denote the forward direction of the reaction. In the above reaction, one molecule of methane and two molecules of oxygen react together and form two molecules of water and one molecule of carbon dioxide.
Look at the following picture of chemical equation
Reactants Products
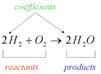
In the picture above, the coefficient of the molecule is shown. Reactants in the given equation are hydrogen and oxygen whereas product is water.
Look at the following chemical equation
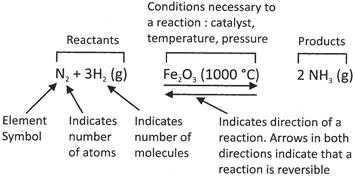
In the above chemical equation, N, is symbolic representation of nitrogen, and 2 in front of N represent the number of atoms. H is the symbolic representation of hydrogen and 3 behind H is representing the number of molecule of hydrogen. In the parenthesis, g represents the gas form of hydrogen. Arrows in the both= direction indicates the reversible reaction. 1000°C (Ferric oxide above the arrow) indicates the necessity of resistive components during the reaction. The product of the reaction is ammonia in the form of gas.
![]() Balancing of Chemical Equations
Balancing of Chemical Equations
The chemical equation is obtained from the chemical experiment. Balancing of chemical equation is defined as the number of atoms in the reactants should be equal to the number of atoms in the resulting products.
Following steps are used for the balancing of the chemical equation:
Step 1: Experimentally determine the reactants and products.
Step 2: Write the unbalanced equation using the formula of reactants and products.
Step 3: Write the balanced equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation.
Let us consider a balanced chemical equation:
![]()
At the right hand side of the given chemical equation has following features,
One molecule of sulfur has 8 atoms.
Each of eight molecules of oxygen have 2 atoms.
Product is sulfur dioxide.
Let us consider an unbalanced chemical equation:
![]() (Ammonia)
(Ammonia)
Balance the number of N by writing 2 in front of ![]()
![]()
Balance H by writing 3 in front of ![]()
Now, the balance chemical equation is written as follows,

![]() What does "aq" means in a chemical equation?
What does "aq" means in a chemical equation?
(a) Solid state of matter
(b) Liquid state of matter
(c) Gaseous state of matter
(d) Aqueous solution
(e) None of these
Answer: (d)
![]() In a chemical equation two or more elements are involved. The elements, which are involved in the chemical equation for the formation new substances, is called...........
In a chemical equation two or more elements are involved. The elements, which are involved in the chemical equation for the formation new substances, is called...........
(a) Products
(b) Reactants
(c) Valance electrons
(d) Covalent bond
(e) None of these
Answer: (b)
![]()
Involved substances in a chemical reaction are called reactants and result is/are called products.
Every substance is made up of atoms and atom is made up of three fundamental particles, i.e. electron, proton and neutron.
So far 119 elements have been introduced. Features of these elements are shown by using periodic table.
During the chemical reaction, elements are combined to form a new substance.
You need to login to perform this action.
You will be redirected in
3 sec
