Method of Testing Acid and Base
Category : 7th Class
The testing of acid is done by indicators, i.e. litmus.
![]() Testing of Add
Testing of Add
It requires two litmus, two beakers and the solution of acid and base. The nature of substance, whether it is acidic or basic is obtained from the colour of litmus. The blue color of litmus turns into red, if the solution ,s acidic. The red color of litmus turns into blue, if the solution is basic.
Look at the following picture of testing of Acid
Blue litmus turns to red Red litmus turns to blue

All acids are aqueous solutions and conducts electricity. The dry cell is filled with acid. It is present inside the small chamber of the cell. The high capacity of battery is also made up of acid. Positive ion of an acid acts as the positive is terminal of the battery and negative ion acts as negative terminal of the battery.
Look at the following picture:
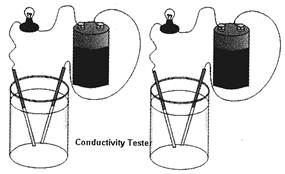
Acid filled jar (A) Base filled jar (B)
In the above picture, jar (A) has acid and an electrical bulb is connected in series. Conducting rod inside the jar is used as a switch. Positive terminal of battery is connected to one end of the bulb and negative terminal of battery is connected to a conducting rod. Another end of the bulb is connected to another rod. Bulb starts glowing, when both rods are into acid and they are not in contact with each other. Therefore, it is clear from the above experiment that current flows through acid.
Read the following about the common indicators and its colour in acid:
| Indicators | Colour in acid |
| Blue litmus | Red |
| Methyl orange | Orange |
| Phenolphthalein | Colorless |
When a metal (Calcium, magnesium, Sodium) is heated in the presence of oxygen a metallic or base oxide is formed.
![]()
(Sodium) (Oxygen) (Sodium Oxide)
Some bases are Iron oxide![]() , Calcium hydroxide
, Calcium hydroxide ![]() Magnesium oxide
Magnesium oxide ![]() aluminum hydroxide
aluminum hydroxide ![]() . etc.
. etc.
Read the Following about the Common Indicators and its Colour In base
| Indicators | Colour in base |
| Red litmus | Blue |
| Methyl orange | Yellow |
| Phenolphthalein | Pink |

![]() The indicator, phenolphthalein turns into which one of the following colour in the acidic solution?
The indicator, phenolphthalein turns into which one of the following colour in the acidic solution?
(a) Red
(b) Black
(c) White
(d) Colorless
(e) None of these
Answer (d)
![]() Which one of the following metals is heated in the presence of oxygen for obtaining sodium oxide?
Which one of the following metals is heated in the presence of oxygen for obtaining sodium oxide?
(a) Copper
(b) Iron
(c) Sodium
(d) Steel
(e) None of these
Answer: (c)
You need to login to perform this action.
You will be redirected in
3 sec
