Acids, Bases and Salts
Category : 7th Class
Learning Objectives
We have learnt in previous class about the classification of substances based on their physical properties like appearance, solubility, hardness etc. Substances can also be grouped on the basis of their taste as sweet, salty, sour or bitter. Have you ever tasted lemon without adding water and salt? Or have you noticed the slippery touch of soap? Lemon juice, tamarind, raw mango etc. taste sour, some substances like baking powder tastes bitter. Common salt tastes salty These tastes are the characteristics of three different types of compounds that are acids, bases and salts. In this section we will learn why some substances are sour or bitter to taste.

Note:- Although all acids taste sour and all bases taste bitter, a taste test is not the best general-purpose way to determine whether a substance is an acid or a base. Some acids and bases are poisonous, and some are quite corrosive. Never taste laboratory chemicals. Too many of them are toxic, and others might be contaminated.
ACIDS
Taste of substances containing acids is sour e.g. curd, lemon juice, orange juice, vinegar, etc. The acids in these substances are natural acids. Lemonade contains citric acid, grapes and tamarind contains tartaric acid. Synthetic substance such as vinegar contains acetic acid and cold drinks contain carbonic acid. The chemical nature of acids is acidic. The word acid comes from Latin word acere which means sour.
Do you know?
Robert boyle was the first to study the properties of acids and described them as sour, corrosive and turning blue litmus red.

Type of Acids
Acids are of two types
Organic acids: These acids contain carbon as a constituent and are present in organic matter i.e, animals and plants. For example Citric acid, acetic acid, tartaric acid etc. Organic acids are weak acids.
Mineral acids: These acids are prepared from minerals present in the Earth's crust. For example sulphuric acid\[\text{(}{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{)}\], hydrochloric acid (HC1), nitric acid (HNO^) etc. These are also called laboratory acids. Mineral acids are strong acids.

Do you know?
Sulphuric acid is known as the king of chemicals.
Do you know?
The earlier name for sulphuric acid was oil of vitriol due to its oily appearance, coined by Iranian alchemist Jabir Ibn Haayan.
Note:- Acids are corrosive in nature. Nitric acid and sulphuric acid can destroy human tissues and can also corrode metals.
Do you know?
Our body produces hydrochloric acid in stomach. This acid makes it possible for the humans as well as animals to digest some strong food contents like meat etc. that otherwise cannot be digested.
Uses of Acids
Hydrochloric acid is used for cleaning sinks and sanitry wares and in textile industry as a bleaching agent.
Sulphuric acid and nitric acid are used in manufacture of fertilizers, paints, explosives etc.
Sulphuric acid is used in the batteries for cars, inverters etc.
Acetic acid is used in preservation of food and for enhancing flavor of food.
Citric acid is used in medicine.
Tartaric acid is used in making baking powder by mixing it with baking soda.
Do you know?
Aqua regia is very powerful acid. This terminology is taken from Latin, it means royal water. It can dissolve gold. It is made of one part nitric acid and three parts of concentrated hydrochloric acid.
Some Acids Present in Commonly Used Substances
|
Name of Acid |
Found in |
|
(i) Acetic acid |
Vinegar |
|
(ii) Formic acid |
Ant’s sting |
|
(iii) Citric acid |
Citrus fruits such as oranges, lemon, etc. |
|
(iv) Lactic acid |
Curd |
|
(v) Oxalic acid |
Spinach |
|
(vi) Ascorbic acid (Vitamin C) |
Amla, Citrus fruits |
|
(vii) Tartaric acid |
Grapes, Unripe mangoes, etc. |
ACID RAIN
The rain containing excess of acids is called acid rain. Rain becomes acidic because carbon dioxide, sulphur dioxide and nitrogen dioxide which are released into air as pollutants dissolve in rain drops to form carbonic acid, sulphuric acid and nitric acid respectively. Acid rain damages buildings, historical monuments, plants and animals.
BASES
Substances which are soapy to touch and are bitter in taste are called bases, e.g., baking soda, washing soda, calcium hydroxide, etc.
Chemical nature of bases is basic.
Bases also occur in plant and animal bodies such as corn starch, fresh egg white.
Do you know?
Blood when healthy is basic in nature. Saliva in our mouth is also basic.
Weak and Strong Bases
Strong bases: Some bases are readily soluble in water. These are strong bases. These are also called alkalies for example: sodium hydroxide and potassium hydroxide.
Weak bases: Some bases are insoluble or partly soluble in water such bases are called weak bases. For example: ammonium hydroxide, calcium hydroxide etc.
Note:- Strong bases are very corrosive and can burn the skin. Uses of Bases
Calcium hydroxide is used as an ingredient in whitewash, neutralizing acidic soil, in making bleaching powder and softening hard water.
Magnesium hydroxide also known as milk of magnesia is used as an antacids.
Sodium hydroxide also known as caustic soda is used manufacture of soaps, paper and textile.
Aluminium hydroxide is used as foaming agent in fire extinguishers.
Ammonium hydroxide is used in household cleaners and in fertilizers.
Sodium carbonate along with sulphuric acid is used in fire extinguisher
Some Bases Present in Commonly Used Substances
|
Name of Base |
Found in |
|
(i) Calcium hydroxide |
Lime water |
|
(ii) Ammonium hydroxide |
Window cleaner |
|
(iii) Sodium hydroxide |
Soap |
|
(iv) Potassium hydroxide |
Soap |
|
(v) Magnesium hydroxide |
Milk of Magnesia |
NEUTRAL SUBSTANCES
Substances which are neither acidic nor basic are called neutral substances. For example solids like sugar, common salt. Liquids like alcohol, benzene, water and gases like hydrogen, nitrogen etc.
INDICATORS
Those substances which are used to test whether a substance is acidic or basic in nature are known as indicators. Natural Indicators some commonly used indicators are
(i) Turmeric
(ii) Litmus
(iii) China rose petals (Gudhal)

These are some naturally occurring indicators. These show different colors in acidic, basic and neutral substances.
Turmeric
Turmeric is the most common indicator available in our homes. It helps in identifying the nature of a substance. The yellow substance in turmeric is called curcumin. A basic substance changes the yellow colour of the turmeric stain to brownish red and an acidic substance reverses the brownish red stain to its original yellow stain.
Neutral substance for example water does not change the yellow colour of mturmeric.

Chinarose
Light pink coloured water obtained from petals of chinarose is used as and indicator. It gives dark pink (magenta) colour in acidic solution and green in basic solutions.
Chinarose indicator remains light pink in neutral medium.
Litmus
It is a natural dye extracted from lichens. It is the most commonly used indicator In distilled water it has a mauve (purple) colour.
In acidic solution it gives a red colour.
In basic solution it gives a blue colour.
It is available as a solution or in strips of paper, known as litmus paper available mas red litmus paper or blue litmus paper.

Olfactory Indicators
There are some natural substances whose odour changes in acidic or basic medium. These are called olfactory indicators, e.g., onion, vanilla and clove oil.
With olfactory indicators different odour can be detected in bases whereas odour remains same in acids.
Acid-base Synthetic Indicators
Phenolphthalein and Methyl orange are commonly used synthetic acid base indicators.
|
Colors of Indicators in Acids and Bases |
||
|
Indicator |
Color in basic medium |
Color in acidic medium |
|
Methyl orange |
Yellow |
Pink |
|
Phenolphthalein |
Pink |
Colorless |
Do you know?
The concept of pH was first introduced by Danish chemist SPL Sorensen in 1909. Hydrangea macrophylla is an ornamental plant. The color of its flowers depends upon the pH of the soil. In acidic soil flower color is blue, in basic soil pink and white in neutral soil.
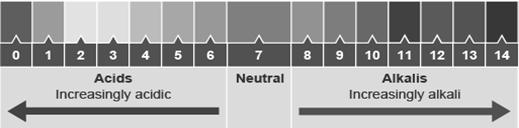
pH
pH scale represents the strength of acid and base. The pH scale varies from 1 to 14. Acidic solution has pH value less than 7, basic solution has pH value between 7 to 14 while neutral solution has pH value 7.
Universal Indicator
A universal indicator is a mixture of indicators that gives a different colour for most of the pH values. The indicator can be used as a liquid or can be soaked into paper. This paper is called pH paper.
Do you know?
Lower the pH value of an acid, the stronger it is. The higher the value of an alkali, the stronger it is.
Do you know?
Human blood has pH of 7.3. Acid rain can has pH of 6.3.
SALTS
Salts are the compounds obtained by neutralization of acid and base in a neutralization reaction.
The most common example of salt is common salt (Sodium chloride). Lime stone (calcium carbonate), soda ash (anhydrous sodium carbonate) etc. are some other example of salts.
Hydrated and Anhydrous Salts
The crystals of some salts have a fixed number of water molecules (called water of crystallization) loosely associated with them such salts are called hydrated salts. These hydrated salts on heating lose their water of crystallization and change to powdery substance called anhydrous salt.
Do you know?
All salts may not be neutral. Some salts are acidic in solution and some salts are basic in solution.
Uses of Salts
Salt absorb moisture from environment without getting dissolved in it. Such property is called hygroscopic. Small clothes bags containing salts (for example calcium chloride) is used in sealed pack of electronic items to keep them dry.
NEUTRALIZATION
The reaction between an acid and a base is known as neutralization. Salt and water are produced in this process with evolution of heat.
\[\text{Acid}+\text{Base}\xrightarrow{{}}\text{Salt}+\text{Water}+\text{Heat}\]
e.g.. \[\underset{(\text{acid})}{\overset{{}}{\mathop{\text{Hydrochloric acid}}}}\,\]+ \[\underset{(\text{Base})}{\overset{{}}{\mathop{\text{Sodium hydroxide}}}}\,\] \[\xrightarrow{{}}\] \[\underset{(\text{salt})}{\overset{{}}{\mathop{\text{Sodium chloride}}}}\,\] + Water
\[\text{HCl+NaOH}\xrightarrow{{}}\text{NaCI+}{{\text{H}}_{\text{2}}}\text{O}\]
In neutralization reaction heat is always evolved. Therefore it is an exothermic reaction.
NEUTRALIZATION IN EVERYDAY LIFE
Indigestion
Our stomach contains hydrochloric acid which helps in digestion of food. If this acid is present in excess quantity in stomach it causes indigestion. Indigestion is painful. To releive indigestion we take antacids such as milk of magnesia which contains magnesium hydroxide as a base. It neutralises the effect of excess acid.

Bee Sting or Ant Bite
When an ant bites or bee stings, it injects acidic liquid (formic acid) into skin. To neutralise the acidic effect we rub moist baking soda (a base) or calcimine solution. Baking Soda contains sodium hydrogen carbonate. Calamine solution contains zinc carbonate.
Soil Treatment
Soil becomes acidic from excessive use of fertilizers. Plants do not grow well when the soil is acidic or basic. If the soil is too acidic it is treated with bases like quick lime (calcium oxide) or slaked lime (calcium hydroxide). In case the soil is basic then we add organic matter which releases acids and neutralises the basic nature of soil.

Bee sting
Factory Waste
Mostly factory wastes contain acids. If these wastes are allowed to enter water bodies the acids will kill fish and other organisms. These wastes are first neutralised by adding basic substances.
Acid Burns
In case of acid bums a dilute solution of baking soda may be sprinkled on the affected body part.
Toothpaste
Toothpaste is alkaline .in nature. This is to neutralize the acid produced by fermentation of food particles in our mouth.
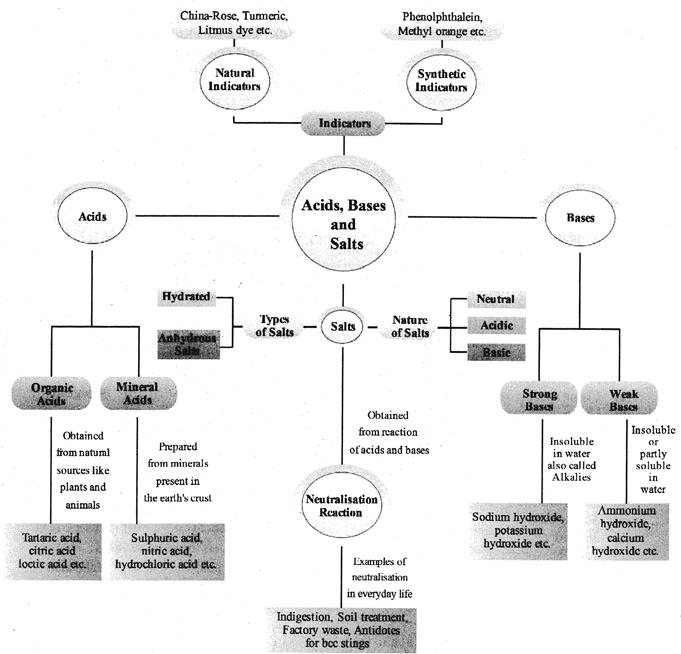
CONCEPT MAP
You need to login to perform this action.
You will be redirected in
3 sec
