MATTER AND MATERIAL
FUNDAMENTAL
Anything that has mass and occupies space is called matter. Amount of matter present in a body is called mass of the body.
Volume is the amount of space which is occupied by the body or an object.
- Whole universe is made up of only two things matter and energy.
Classification of Matter
|
On physical basis
|
Chemical basis
|
|
1. Solid e.g.
|
1. Element\[\to \]sodium, potassium.
|
|
2. Liquid and water
|
2. Compound\[\to \]water, CO2
|
|
3. Gas-Hydrogen, oxygen
|
3. Mixture\[\to \]sugar solid, salt, solution
|
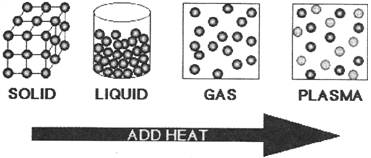
Matters exist in three states-solids, liquids or gases.
- Matter is made up of tiny particles called molecule.
- In solids, attraction between molecules is very strong solids have fixed shape, size and volume.
- In liquid the attraction between molecules is not very strong. Liquids don't have a fixed shape and can flow freely.
- In gas, the attraction between molecules is very week so gas molecules move freely. They don't have a fixed shape or size and volume.
- One form of matter can be changed to another form. Solid melts to form liquids change into gases.
- When a solute is dissolved in a solvent a solution is formed
- Water is one of the few known substances which expand when it freezes. Thus ice occupies more space than water.
- The increase in size of the matter on heating is called expansion.
- The decrease in the size of the matter on cooling is called contraction. Solution can be homogenous or heterogeneous. Homogenous solution, the solute dissolves uniformly in the solvent so that the solutes can't be seen through naked eye. Heterogamous solutions - solutes doesn't dissolve uniformly, so that solute particles are visible.
- Solutes, when dissolves in a liquid, occupies the empty space present between the molecules. If all the empty spaces are occupied by solute and no space is left between the solvent molecules, it is called saturated solution, i.e., no more solutes can be added to a given amount of solvent. If more solute is added. It settles at the bottom. Saturated solutions solubility can be increased by increasing the temperature of the solution.

