Purification And Characterisation Of Organic Compounds
Category : JEE Main & Advanced
The study of organic compounds starts with the characterisation of the compound and the determination of its molecular structure. The procedure generally employed for this purpose consists of the following steps :
(1) Purification of organic compounds
(2) Qualitative analysis of organic compounds
(3) Quantitative analysis of organic compounds
(4) Determination of molecular mass of organic compounds
(5) Calculation of Empirical formula and Molecular formula of organic compounds
(6) Determination of structure of organic compounds by spectroscopic and diffraction methods
(1) Purification of organic compounds : A large number of methods are available for the purification of substances. The choice of method, however, depends upon the nature of substance (whether solid or liquid) and the type of impurities present in it. Following methods are commonly used for this purpose,
(i) Simple crystallisation
(ii) Fractional crystallisation,
(iii) Sublimation
(iv) Simple distillation
(v) Fractional distillation
(vi) Distillation under reduced pressure
(vii) Steam distillation
(viii) Azeotropic distillation
(ix) Chromatography
(x) Differential extraction
(xi) Chemical methods
(i) Simple crystallisation : This is the most common method used to purify organic solids. It is based upon the fact that whenever a crystal is formed, it tends to leave out the impurities. For crystallisation, a suitable solvent is one (a) which dissolves more of the substance at higher temperature than at room temperature (b) in which impurities are either insoluble or dissolve to an extent that they remain in solution (in the mother liquor) upon crystallisation, (c) which is not highly inflammable and (d) which does not react chemically with the compound to be crystallized. The most commonly used solvents for crystallisation are : water, alcohol, ether, chloroform, carbon- tetrachloride, acetone, benzene, petroleum ether etc.
Examples : (a) Sugar having an impurity of common salt can be crystallized from hot ethanol since sugar dissolves in hot ethanol but common salt does not.
(b) A mixture of benzoic acid and naphthalene can be separated from hot water in which benzoic acid dissolves but naphthalene does not.
(ii) Fractional crystallisation : The process of separation of different components of a mixture by repeated crystallisations is called fractional crystallisation. The mixture is dissolved in a solvent in which the two components have different solubilities. When a hot saturated solution of this mixture is allowed to cool, the less soluble component crystallises out first while the more soluble substance remains in solution. The mother liquor left after crystallisation of the less soluble component is again concentrated and then allowed to cool when the crystals of the more soluble component are obtained. The two components thus separated are recrystallized from the same or different solvent to yield both the components of the mixture in pure form.
Fractional crystallisation can be used to separate a mixture of \[KCl{{O}_{3}}\](less soluble) and KCl (more soluble).
(iii) Sublimation : Certain organic solids on heating directly change from solid to vapour state without passing through a liquid state, such substances are called sublimable and this process is called sublimation.
![]()
The sublimation process is used for the separation of sublimable volatile compounds from non sublimable impurities. The process is generally used for the purification of camphor, naphthalene, anthracene, benzoic acid \[N{{H}_{4}}Cl,\,HgC{{l}_{2}}\], solid \[S{{O}_{2}}\], Iodine and salicylic acid etc containing non-volatile impurities.
(iv) Simple distillation : Distillation is the joint process of vapourisation and condensation. This method is used for the purification of liquids which boil without decomposition and contain non-volatile impurities. This method can also be used for separating liquids having sufficient difference in their boiling points. This method can be used to separate a mixture of
(a) chloroform (b. p. 334 K) and aniline (b. p. 457 K)
(b) ether (b. p. 308 K) and toluene (b. p. 384 K)
(v) Fractional distillation : This process is used to separate a mixture of two or more miscible liquids which have boiling points close to each other. Since in this process, the distillate is collected in fractions under different temperatures, it is known as fractional distillation. This process is carried out by using fractionating columns. Fractionating column is a special type of long glass tube provided with obstructions to the passage of the vapour upwards and that of liquid downwards. This method may be used to separate a mixture of acetone (b. p. 330 K) and methyl alcohol (b. p. 338 K) or a mixture of benzene and toluene. One of the technological applications of fractional distillation is to separate different fractions of crude oil in petroleum industry into various useful fractions such as gasoline, kerosene oil, diesel oil, lubricating oil etc.
(vi) Distillation under reduced pressure : This method is used for the purification of high boiling liquids and liquids which decompose at or below their boiling points.
The crude liquid is heated in distillation flask fitted with a water condenser, receiver and vacuum pump. As the pressure is reduced, the liquid begins to boil at a much lower temperature than its normal boiling point. The vapour is condensed by water condenser and the pure liquid collects in the receiver.
Glycerol which decomposes at its boiling point (563 K) under atmospheric pressure can be distilled without decomposition at 453 K under 12 mm of Hg. Similarly, sugarcane juice is concentrated in sugar industry by evaporation under reduced pressure which saves a lot of fuel.
(vii) Steam distillation : This method is applicable for the separation and purification of those organic compounds (solids or liquids) which (a) are insoluble in water (b) are volatile in steam (c) possess a high vapour pressure (10-15 mm Hg) at 373 K and (d) contain non-volatile impurities.
Aniline (b. p. 457 K) can be purified by steam distillation since it boils at a temperature of 371.5 K in presence of steam. Other compounds which can be purified by steam distillation are: nitrobenzene, bromobenzene, o-nitrophenol, salicylaldehyde, o-hydroxyacetophenone, essential oils, turpentine oil etc.
(viii) Azeotropic distillation : Azeotropic mixture is a mixture having constant boiling point. The most familiar example is a mixture of ethanol and water in the ratio of 95.87 : 4.13 (a ratio present in rectified spirit). It boils at 78.13oC. The constituents of an azeotropic mixture can't be separated by fractional distillation. Hence a special type of distillation (azeotropic distillation) is used for separating the constituents of an azeotropic mixture.
In this method a third compound is used in distillation. The process is based on the fact that dehydrating agents like \[{{C}_{6}}{{H}_{6,\,\,\,}}CC{{l}_{4}}\], diethyl ether etc. depress the partial pressure of one of the original components. As a result, the boiling point of that component is raised sufficiently and thus the other component will distil over.
Dehydrating agents having low boiling point (e.g. \[{{C}_{6}}{{H}_{6,\,\,}}CC{{l}_{4,}}\]ether) depress the partial pressure of alcohol more than that of water; on the other hand, dehydrating agents having high boiling point (glycerol, glycol) depress the partial pressure of water more than that of alcohol.
(ix) Chromatography : This is a modern method used for the separation of mixtures into its components, purification of compounds and also to test the purity of compounds. The name chromatography is based on the Greek word 'chroma' meaning colour and 'graphy' for writing because the method was first used for the separation of coloured substances found in plants. This method was described by Tswett in 1906.
(a) Principle of chromatography : The technique of chromatography is based on the difference in the rates at which the components of a mixture move through a porous medium (called stationary phase) under the influence of some solvent or gas (called moving phase). Thus, this technique consists of two phases- one is a stationary phase of large surface area while the second is a moving phase which is allowed to move slowly over the stationary phase. The stationary phase is either a solid or a liquid while the moving phase may be a liquid or a gas.
(b) Types of chromatography : Depending upon the nature of the stationary and the mobile phases, the different types of chromatographic techniques commonly used are in a given table,
| Type of Chromatography | Mobile/Stationary Phase | Uses |
| Adsorption or column chromatography | Liquid/Solid | Large scale separations |
| Thin-layer chromatography | Liquid/Solid | Qualitative analysis (identification and characterization of organic compounds) |
| High performance liquid chromatography | Liquid/Solid | Qualitative and quantitative analysis |
| Gas-liquid chromatography (GLC) | Gas/Liquid | Qualitative and quantitative analysis |
| Partition chromatography or ascending paper chromatography | Liquid/Liquid | Qualitative and quantitative analysis of polar organic compounds (sugars, a-amino acids and inorganic compounds) |
It is constant for a given substance (component) under a given set of conditions. Therefore, it is possible to identify the various components by determining their \[{{R}_{f}}\] values.
(x) Differential extraction : This method is used for the separation of an organic compound (solid or liquid) from its aqueous solution by shaking with a suitable solvent (e.g. ether, benzene, chloroform, carbon tetrachloride etc.) in a separating funnel. The selected solvent should be immiscible with water but should dissolve the organic compound to an appreciable extent.
It is important to note that extraction is more efficient (i.e., more complete) when a given volume of the extracting solvent is used in several installments.
This method is normally applied to nonvolatile compounds. For example, benzoic acid can be extracted from its water solution using benzene.
(xi) Chemical methods : Besides these physical methods, a number of chemical methods have also been used to separate a mixture of organic compounds. These methods are based upon the distinguishing chemical properties of one class of organic compounds from the others. For example,
(a) Phenols can be separated from carboxylic acids on treatment with an aqueous solution of \[NaHC{{O}_{3}}\]. Since carboxylic acids dissolve in \[NaHC{{O}_{3}}\]solution evolving \[C{{O}_{2}}\] but phenols usually do not react.
(b) Destructive distillation of wood gives pyroligneous acid which contains acetic acid (10%), acetone (0.5%) and methanol (3%). Acetic acid can be separated from this mixture by treating it with milk of lime when acetic acid forms the calcium salt. The reaction mixture on distillation gives a mixture of acetone and methanol (which can be further separated by fractional distillation into individual components as mentioned above) while the calcium salt remains as residue in the flask. The calcium salt is then decomposed with dil HCl and distilled to afford acetic acid.
(c) A mixture of 1o, 2o and 3o amines can be separated using either benzenesulphonyl chloride (Hinsberg's reagent) or diethyl oxalate (Hoffmann's method).
(d) Purification of commercial benzene : Commercial benzene obtained from coal-tar distillation contains 3-5% thiophene as an impurity which can be removed by extraction with conc. \[{{H}_{2}}S{{O}_{4}}\]. This purification is based upon the fact that thiophene undergoes sulphonation much more easily than benzene. Thus, when commercial benzene is shaken with conc. \[{{H}_{2}}S{{O}_{4}}\] in a separating funnel, thiophene undergoes sulphonation to form thiophene-2-sulphonic acid which dissolves in conc. \[{{H}_{2}}S{{O}_{4}}\] while benzene does not.
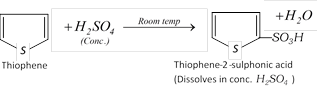
After this treatment, the benzene layer is removed, washed with water to remove unreacted \[{{H}_{2}}S{{O}_{4}}\], dried over anhyd. \[CaC{{l}_{2}}\] and then distilled to give pure benzene.
(e) Absolute alcohol from rectified spirit : The rectified spirit (ethanol : \[{{H}_{2}}O,\,95.\,87\,:\,4.13\] by weight) is kept over a calculated amount of active quick lime (CaO) for few hours and then refluxed. During this process, water present in rectified spirit combines with CaO to form \[Ca{{(OH)}_{2}}\]. When the resulting mixture is distilled, absolute alcohol distils over leaving behind, \[Ca{{(OH)}_{2}}\].
Drying of Organic Substances. (1) For solids : Most solids are dried first by pressing them gently between folds of filter papers. Compounds which neither decompose on heating nor melt below 100oC are dried by keeping them in steam or oven maintained at 110oC. Substances, which decompose on heating are dried by keeping them in a vacuum desiccator containing a suitable dehydrating agent like fused \[CaC{{l}_{2}}\], conc. \[{{H}_{2}}S{{O}_{4}}\], \[{{P}_{4}}{{O}_{10}},\] solid KOH or NaOH, etc (desiccant).
(2) For liquids : Organic liquids are generally dried by keeping them over night in contact with a dehydrating (desiccating) agent which does not react chemically with the liquid to be dried. Commonly used dehydrating agents are quick lime, anhydrous \[CaC{{l}_{2}}\], fused \[CuS{{O}_{4}}\]or \[CaS{{O}_{4}},KOH\], metallic sodium or potassium, etc.
Criteria of purity of organic compounds : The purity of an organic compound can be ascertained by determining its some physical constants like m.p., b.p., specific gravity, refractive index and viscosity. In usual practice, sharp m.p. (in case of solids) and boiling point (in case of liquids) are used as criteria for purity because their determination is feasible in the laboratory. A pure organic solid has a definite and sharp (sudden, rapid and complete) melting point, while an impure substance has a lower and indefinite melting point.
(1) Mixed melting point : The melting point of two thoroughly mixed substances is called mixed melting point. This can also be used for ascertaining the purity of a compound .
The substance, whose purity is to be tested, is mixed with a pure sample of the same compound. The melting point of the mixture is determined. If the melting point of the mixture is sharp and comes out to be the same as that of pure compound, it is sure that the compound under test is pure. On the other hand, if the melting point of the mixture is less than the melting point of the pure compound, the compound in question is not pure.
(2) Qualitative analysis : (Detection of Elements )
The qualitative analysis of an organic compound involves the detection of all the elements present in it.
Carbon is an essential constituent of an organic compound whereas hydrogen is nearly always present. On heating the organic compound with dry cupric oxide, carbon is oxidized to \[C{{O}_{2}}\] and hydrogen to \[{{H}_{2}}O\]. \[C{{O}_{2}}\] is detected by lime water which turns milky while \[{{H}_{2}}O\] is detected by anhydrous \[CuS{{O}_{4}}\] (white) which turns it blue. This method is known as copper oxide test.
\[\underset{{}}{\mathop{C}}\,+2CuO\xrightarrow{\text{Heat}}C{{O}_{2}}+2Cu\] ;
\[\underset{\text{Lime water}}{\mathop{Ca{{(OH)}_{2}}}}\,+C{{O}_{2}}\xrightarrow{{}}\underset{\text{Milky}}{\mathop{CaC{{O}_{3}}}}\,+{{H}_{2}}O\]
\[{{H}_{2}}+CuO\xrightarrow{\text{Heat}}{{H}_{2}}O+Cu\] ;
\[\underset{\begin{smallmatrix} \,\,\text{Colourles}s \\ \text{(Anhydrous)} \end{smallmatrix}} {\mathop{CuS{{O}_{4}}}}\,+5{{H}_{2}}O\xrightarrow{{}}\underset{\begin{smallmatrix} \,\,\,\,\,\text{Blue } \\ \text{(Hydrated)} \end{smallmatrix}}{\mathop{CuS{{O}_{4}}.5{{H}_{2}}O}}\,\]
If the substance under investigation is a volatile liquid or gas, the vapours are passed over heated copper oxide kept in combustion tube and the gaseous products are tested as above.
Lassaigne method
This is used to detect nitrogen, halogen and sulphur. Organic compounds are fused with dry sodium in a fusion-tube and fused mass after extraction with \[{{H}_{2}}O\] is boiled and filtered. Filtrate called sodium extract (S.E.) is used to detect elements (other than C and H) and the tests are given in the table.
| Element | Sodium Extract (S.E.) | Confirmed Test | Reaction |
| Nitrogen | \[Na+C+N\xrightarrow{\Delta }\underset{(S.E.)}{\mathop{NaCN}}\,\] | S.E.+\[FeS{{O}_{4}}+NaOH\], boil and cool + \[FeC{{l}_{3}}+conc.HCl\] Blue or green colour | \[2NaCN+FeS{{O}_{4}}\xrightarrow{{}}Fe{{(CN)}_{2}}+N{{a}_{2}}S{{O}_{4}}\] \[Fe{{(CN)}_{2}}+4NaCN\xrightarrow{{}}\underset{\text{Sodium ferrocyanide}}{\mathop{N{{a}_{4}}[Fe{{(CN)}_{6}}]}}\,\] \[3N{{a}_{4}}[Fe{{(CN)}_{6}}]+4FeC{{l}_{3}}\xrightarrow{HCl}\underset{\begin{smallmatrix} \text{Ferric ferrocyanide} \\ \,\,\,\,\,\,(\text{Prussian blue)} \end{smallmatrix}}{\mathop{F{{e}_{4}}{{[Fe{{(CN)}_{6}}]}_{3}}}}\,+12NaCl\] |
| Sulphur | \[2Na+S\xrightarrow{\Delta }\underset{\text{(S}\text{.E}\text{.)}}{\mathop{N{{a}_{2}}S}}\,\] | (i) S.E. + sodium nitro prusside (ii)S.E+\[C{{H}_{3}}C{{O}_{2}}H+{{(C{{H}_{3}}C{{O}_{2}})}_{2}}Pb\] A black ppt. |
(i) \[\underset{\ \ \ \ \ \ \ \ \ \ \ \ \text{Sodium nitroprusside}}{\mathop{N{{a}_{2}}S+N{{a}_{2}}[Fe{{(CN)}_{5}}NO]}}\,\xrightarrow{{}}\underset{\text{(Purple)}}{\mathop{N{{a}_{4}}[Fe{{(CN)}_{5}}NO.S]}}\,\] or \[\underset{\begin{smallmatrix} \text{Sodium thionitroprusside} \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(\text{Violet)} \end{smallmatrix}}{\mathop{N{{a}_{3}}[Fe(ONSNa){{(CN)}_{5}}]}}\,\] (ii) \[N{{a}_{2}}S+{{(C{{H}_{3}}COO)}_{2}}Pb\xrightarrow{C{{H}_{3}}COOH}\underset{black\,ppt.}{\mathop{PbS\downarrow }}\,+2C{{H}_{3}}COONa\] |
| Halogen | \[Na+X\xrightarrow{\Delta }\underset{(S.E.)}{\mathop{NaX}}\,\] (X = Cl, Br, I) | S.E. \[+HN{{O}_{3}}+AgN{{O}_{3}}\] (i) White ppt soluble in aq \[N{{H}_{3}}\] confirms \[Cl\]. (ii) Pale yellow ppt partially soluble in aq. \[N{{H}_{3}}\] confirms Br. (iii) Yellow ppt insoluble in aq \[N{{H}_{3}}\] confirms I. | \[NaX+AgN{{O}_{3}}\xrightarrow{HN{{O}_{3}}}\underset{ppt}{\mathop{AgX\downarrow }}\,\] \[\underset{\text{White}\,\text{ppt}}{\mathop{AgCl}}\,+2N{{H}_{3}}(aq)\xrightarrow{{}}\underset{\text{soluble}}{\mathop{[Ag{{(N{{H}_{3}})}_{2}}]Cl}}\,\] \[\underset{\text{Yellow}\ \text{ppt}\text{.}}{\mathop{AgBr}}\,+2N{{H}_{3}}(aq)\to \underset{\text{Partially soluble }}{\mathop{[Ag{{(N{{H}_{3}})}_{2}}]Br}}\,\] \[AgI+N{{H}_{3}}(aq)\xrightarrow{{}}\text{Insoluble}\] |
| Nitrogen and sulphur together | \[Na+C+N+S\xrightarrow{\Delta }\underset{\text{(S}\text{.E}\text{.)}}{\mathop{NaCNS}}\,\]with excess of Na the thiocyanate formed decomposes into cyanide and sulphide. \[NaCNS+2Na\to NaCN\] \[+N{{a}_{2}}S\] | As in test for nitrogen; instead of green or blue colour, blood red colouration confirms presence of \[N\] and \[S\] both. | \[3NaCNS+FeC{{l}_{3}}\xrightarrow{{}}\underset{\begin{smallmatrix} \text{Ferric sulphocyanide} \\ \,\,\,\,\,\,(\text{Blood red colour) } \end{smallmatrix}}{\mathop{[Fe{{(SCN)}_{3}}\ \text{or}\ [Fe(SCN)]C{{l}_{2}}}}\,+3NaCl\] |
Other methods for detection of elements
| Element | Test |
| Nitrogen | Soda lime test : A pinch of an organic compound is heated strongly with soda lime \[(NaOH+CaO)\] in a test tube. If ammonia gas evolves, it indicates nitrogen. \[\underset{\text{Acetamide}}{\mathop{C{{H}_{3}}CON{{H}_{2}}}}\,+NaOH\xrightarrow{CaO}C{{H}_{3}}COONa+N{{H}_{3}}\]. This test is, however, not reliable since certain compounds like nitro, azo etc do not evolve \[N{{H}_{3}}\]when heated with soda lime. |
| Sulphur | Oxidation test : Sulphur can also be tested by oxidation test. The organic compound is fused with fusion mixture (a mixture of sodium carbonate and potassium nitrate). The sulphur, if present in the organic compound, is oxidised to sodium sulphate. \[N{{a}_{2}}C{{O}_{3}}+S+3O\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+C{{O}_{2}}\]. The fused mass is dissolved in water and the solution is acidified with hydrochloric acid. Barium chloride solution is then added. The formation of a white precipitate indicates the presence of sulphur. \[N{{a}_{2}}S{{O}_{4}}+BaC{{l}_{2}}\xrightarrow{{}}\underset{\text{(White ppt}\text{.)}}{\mathop{BaS{{O}_{4}}}}\,+2NaCl\]. |
| Halogens | Beilstein's test (copper wire test) : A clean copper wire is heated in the Bunsen flame till it does not impart any green colour to the flame. The heated end is dipped in the organic compound and heated again. The appearance of a green or bluish green flame due to the formation of volatile cupric halides indicates the presence of some halogen in the organic compound. Though this test is very sensitive yet it does not confirm the presence of halogens in an organic compound since certain organic compounds like urea, thiourea, pyridine, organic acids etc. which do not contain halogens give this test due to the formation of volatile cupric cyanide. It does not tell as to which halogen is present. Special test for bromine and iodine (layer test) : Boil a portion of the Lassaigne's extract with nitric acid. Add a few drops of \[C{{S}_{2}}\]and then add chlorine water slowly with constant shaking. An orange colouration in \[C{{S}_{2}}\] layer confirms the presence of bromine where as a violet colouration in the layer confirms the presence of iodine. \[2NaBr+C{{l}_{2}}\xrightarrow{{}}\underset{\text{tums C}{{\text{S}}_{\text{2}}}\,\text{layer orange}}{\mathop{2NaCl+B{{r}_{2}}}}\,\]; \[2NaI+C{{l}_{2}}\xrightarrow{{}}\underset{\text{turns C}{{\text{S}}_{\text{2}}}\,\text{layer}\,\text{violet}}{\mathop{2NaCl+{{I}_{2}}}}\,\] |
| Phosphorus | Phosphorus is detected by fusing the organic compound with sodium peroxide when phosphorus is converted into sodium phosphate. \[2P+5N{{a}_{2}}{{O}_{2}}\xrightarrow{{}}2N{{a}_{3}}P{{O}_{4}}+2N{{a}_{2}}O\]. The fused mass is extracted with \[{{H}_{2}}O\], boiled with conc. \[HN{{O}_{3}}\] and then ammonium molybdate is added. Appearance of yellow ppt. or colouration due to the formation of ammonium phosphomolybdate indicates the presence of phosphorus. \[N{{a}_{3}}P{{O}_{4}}+3HN{{O}_{3}}\xrightarrow{\Delta }{{H}_{3}}P{{O}_{4}}+3NaN{{O}_{3}}\] \[{{H}_{3}}P{{O}_{4}}+\underset{\text{Amm}\text{. molybdate}}{\mathop{12{{(N{{H}_{4}})}_{2}}Mo{{O}_{4}}}}\,+21HN{{O}_{3}}\xrightarrow{{}}\underset{\begin{smallmatrix} \text{Amm}\text{. Phosphomolybdate} \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{(yellow ppt}\text{.)} \end{smallmatrix}}{\mathop{{{(N{{H}_{4}})}_{3}}P{{O}_{4}}.12Mo{{O}_{3}}}}\,+21N{{H}_{4}}N{{O}_{3}}+12{{H}_{2}}O\] |
| Oxygen | There is no satisfactory qualitative method for the detection of oxygen. However, its presence can be inferred indirectly. (i) If the organic compound is heated alone in a dry test tube in the presence of nitrogen, the formation of water drops on cooler parts of the tube may indicate the presence of oxygen. (ii) The presence of oxygen can be inferred by testing the presence of functional groups known to contain oxygen, e.g., hydroxyl (?OH), aldehydic (?CHO), carboxyl (?COOH) groups, etc. |
(3) Quantitative analysis (Estimation of Elements) : After qualitative analysis of elements, the next step in the determination of molecular formula of an organic compound is the estimation of various elements by mass, i.e. finding the percentage composition of the elements by mass. The various methods commonly employed for the estimation of principal elements are discussed in the table. Quantitative estimation of elements in organic compounds
| Element | Method and its principle | Formula |
| Carbon and Hydrogen | Liebig's combustion method : In this method, a known weight of organic compound is heated with pure and dry cupric oxide in a steam of pure and dry oxygen, when carbon is oxidised to carbon dioxide while hydrogen is oxidised to water. From the weight of \[C{{O}_{2}}\]and \[{{H}_{2}}O\], the percentage of C and H can be calculated. \[{{C}_{x}}{{H}_{y}}+\left( x+\frac{y}{4} \right){{O}_{2}}\xrightarrow{\Delta }xC{{O}_{2}}+\frac{y}{2}{{H}_{2}}O\] | (i) \[\text{ }\!\!%\!\!\text{ of }C=\frac{\text{Weight of }C{{O}_{2}}}{\text{Weight of org}\text{. compound}}\times \frac{12}{44}\times 100\] (ii) \[\text{ }\!\!%\!\!\text{ of }H=\frac{\text{Weight of }{{H}_{2}}O}{\text{Weight of org}\text{. compound}}\times \frac{2}{18}\times 100\] |
| Nitrogen | (i) Duma's method : Elemental nitrogen is converted into molecular nitrogen by a suitable chemical method and its voiume is changed to STP data. \[C+2H+3CuO\to C{{O}_{2}}+{{H}_{2}}O+3Cu\] \[2N+2CuO\to {{N}_{2}}+\]oxide of nitrogen \[\text{Oxides of nitrogen }+\text{ }Cu\xrightarrow{{}}{{N}_{2}}+CuO\] (ii) Kjeldahl's method : Nitrogen in organic compound is converted into \[N{{H}_{3}}\] by suitable chemical method which, in turn, is absorbed by \[{{V}_{1}}mL\] of \[{{N}_{1}}{{H}_{2}}S{{O}_{4}}\]. \[N(\text{from organic compound})+\text{ conc}\text{. }{{H}_{2}}S{{O}_{4}}\xrightarrow{\Delta }{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\] \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}+2NaOH\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+2{{H}_{2}}O+2N{{H}_{3}}\] | % of N = \[\frac{28}{22400}\times \frac{V}{W}\times 100\] Where, V= volume of \[{{N}_{2}}\]in nitrometer (in ml) at NTP, W= Weight of substance taken; \[\text{ }\!\!%\!\!\text{ of }N\text{ }=\frac{\text{1}\text{.4}\times N\times V}{W}\] Note : This method is, however, not applicable to compounds containing nitrogen in the ring (e.g. Pyridine, quinoline etc) and compounds containing nitro and azo (? N = N ?) groups since nitrogen in these compounds is not completely converted into \[{{(N{{H}_{4}})}_{2}}S{{O}_{4}}\] during digestion. |
| Halogens | (i) Carius method : The method is based on the fact that when an organic compound containing halogen (Cl, Br, or I) is heated in a sealed tube with fuming nitric acid in presence of silver nitrate, silver halide is formed. From the mass of silver halide formed, the percentage of the halogen can be calculated. | \[\text{ }\!\!%\!\!\text{ of }Cl\text{ }=\text{ }\frac{\text{35}\text{.5}}{\text{143}\text{.5}}\times \frac{\text{Mass of }AgCl \text{formed}}{\text{Mass of substance taken }}\times 100\] \[\text{ }\!\!%\!\!\text{ of }Br\text{ }=\text{ }\frac{\text{80}}{\text{188}}\times \frac{\text{Mass of }AgBr \text{formed}}{\text{Mass of substance taken }}\times 100\] \[\text{ }\!\!%\!\!\text{ of }I\text{ }=\text{ }\frac{\text{127}}{\text{235}}\times \frac{\text{Mass of }Agl \text{formed}}{\text{Mass of substance taken }}\times 100\] |
| (ii) Schiff's and Piria method : In this method the accurately weighed organic compound (0.15 ? 0.25 g) is taken in a small platinum crucible with a mixture of lime and sodium carbonate, \[(CaO+N{{a}_{2}}C{{O}_{3}})\]. It is now heated strongly and then cooled and dissolved in dilute nitric acid in a beaker. The solution is then filtered and the halide is precipitated with silver nitrate solution. Halogen is now calculated as in Carius method. | ||
| Sulphur | Carius method : When an organic compound containing sulphur is heated with fuming nitric acid, sulphur is oxidised to sulphuric acid. This is precipitated as barium sulphate by adding barium chloride solution. From the amount of barium sulphate, percentage of sulphur can be calculated. \[S+HN{{O}_{3}}\text{(fuming)}\xrightarrow{\text{heat}}{{H}_{2}}S{{O}_{4}}\] \[{{H}_{2}}S{{O}_{4}}+BaC{{l}_{2}}\xrightarrow{{}}\underset{\text{white ppt}}{\mathop{BaS{{O}_{4}}}}\,+2HCl\] | \[\text{ }\!\!%\!\!\text{ of }S\text{ }=\frac{\text{32}}{\text{233}}\times \frac{\text{Mass of }BaS{{O}_{4}}\text{ formed }}{\text{Mass of substance taken }}\times 100\] |
| phosphorous | Carius method : The organic compound containing phosphorus is heated with fuming nitric acid. Phosphorus is oxidised to phosphoric acid. It is precipitated as magnesium ammonium phosphate, \[MgN{{H}_{4}}P{{O}_{4}}\], by the addition of magnesia mixture \[(MgS{{O}_{4}}+N{{H}_{4}}OH+N{{H}_{4}}Cl\]). The magnesium ammonium phosphate is washed, dried and ignited when it is converted to magnesium pyrophosphate \[(M{{g}_{2}}{{P}_{2}}{{O}_{7}})\]. \[2MgN{{H}_{4}}P{{O}_{4}}\xrightarrow{heat}M{{g}_{2}}{{P}_{2}}{{O}_{7}}+2N{{H}_{3}}+{{H}_{2}}O\] From the mass of magnesium pyro-phosphate, the percentage of phosphorus in the compound can be calculated. | \[\text{ }\!\!%\!\!\text{ of P }=\frac{\text{62}}{\text{222}}\times \frac{\text{Mass of }M{{g}_{2}}{{P}_{2}}{{O}_{7}}\text{ formed }}{\text{Mass of substance taken}}\times 100\] |
| Oxygen | (i) The usual method of determining the percentage of oxygen in an organic compound is by the method of difference. All the elements except oxygen present in the organic compound are estimated and the total of their percentages subtracted from 100 to get the percentage of oxygen. (ii) Aluise's method :. Organic compound containing oxygen is heated with graphite and CO formed is quantitatively converted into CO2 on reaction with I2O5. \[\text{Org}\text{. compound}\xrightarrow{\text{Pyrolysis}}\text{Oxygen}\] \[{{O}_{2}}+2C\xrightarrow{{{1100}^{o}}C}2CO\] \[5CO+{{I}_{2}}{{O}_{5}}\xrightarrow{{}}{{I}_{2}}+5C{{O}_{2}}\] | Percentage of oxygen = 100 - (Sum of the percentages of all other elements) \[\underset{16\,g\,\,\,\,\,\,}{\mathop{\,\,\,\,O\equiv CO}}\,\underset{\,\,\,\,44\,g}{\mathop{\equiv C{{O}_{2}}}}\,\] \[\text{ }\!\!%\!\!\text{ of }O\text{ }=\frac{\text{16}}{\text{44}}\times \frac{\text{mass of C}{{\text{O}}_{\text{2}}}}{\text{mass of org}\text{. compd}\text{.}}\times 100\]. |
(4) Determination of Molecular Mass : The molecular mass of the organic compounds can be determined by various methods.
(i) Physical methods for volatile compounds
(a) Victor Meyer's method : Molecular mass of volatile liquids and solids can be easily determined from the application of Avogadro hypothesis according to which the mass of 22.4 litres or 22400ml of the vapour of any volatile substance at NTP is equal to the molecular mass of the substance.
In Victor Meyer's method, a known mass of the volatile substance is vaporised in a Victor Meyer's tube. The vapours formed displace an equal volume of air into a graduated tube. The volume of air collected in graduated tube is measured under experimental conditions. This volume is converted to NTP conditions.
Calculations : Mass of the organic substance \[=W\,g\]
Let the volume of the air displaced be \[={{V}_{1}}\,ml\];
Temperature \[={{T}_{1}}K\]
Pressure (after deducting aqueous tension) \[={{p}_{1}}mm\]
Let the volume at NTP be \[={{V}_{2}}\,ml\]
Applying gas equation, \[{{V}_{2}}=\frac{{{p}_{1}}\times {{V}_{1}}}{{{T}_{1}}}\times \frac{273}{760}\]
\[\because \] \[{{V}_{2}}\] ml of vapours weight at NTP = Wg
\[\therefore \] 22400 ml of vapour weight at NTP = \[\frac{W}{{{V}_{2}}}\times 22400=M\]
Alternatemethod : Vapour density of substance
\[=\frac{\text{Mass of 1 ml of vapours at NTP}}{\text{Mass of 1 ml of hydrogen at NTP}}\]
or V. D. \[=\frac{W/{{V}_{2}}}{0.00009}\] (\[\because \] Mass of 1 ml of \[{{H}_{2}}\] at NTP
\[=0.00009\,g\] or \[2/22400\])
or V. D. \[=\frac{W}{{{V}_{2}}\times 0.00009}\];
Mol. Mass, \[M=2\times V.D.=\frac{2W}{{{V}_{2}}\times 0.00009}\]
(b) Hofmann's method : The method is applied to those substances which are not stable at their boiling points, but which may be volatilised without decomposition under reduced pressure. A known mass of the substance is vaporised above a mercury column in a barometric tube and the volume of the vapour formed is recorded. It is then reduced to NTP conditions. The molecular mass of the organic substance can be calculated by the application of following relationship,
Mol. Mass \[=\frac{\text{Mass of the substance }}{\text{volume of the vapours at NTP}}\times 22400\]
(ii) Physical methods for Non-volatile substances : The molecular mass of a non-volatile organic compound can be determined by noting either the elevation in boiling point of the solvent (Ebullioscopic method) or the depression in freezing point of the solvent (Cryoscopic method) produced by dissolving a definite mass of the substance in a known mass of the solvent. The molecular mass of the compound can be calculated from the following mathematical relationships :
(a) Elevation in boiling point : Mol. Mass \[=\frac{1000\,{{K}_{b}}\times w}{W\times \Delta T}\]
Where, \[{{K}_{b}}=\]Molal elevation constant of the solvent, \[w=\] Mass of the compound, \[W=\] Mass of the solvent
\[\Delta T=\]Elevation in boiling point of the solvent (determined experimentally)
(b) Depression in freezing point : Mol. Mass \[=\frac{1000\,{{K}_{f}}\times w}{W\times \Delta T}\]
Where, \[{{K}_{f}}=\]Molal depression constant of the solvent, \[w=\] Mass of the compound, \[W=\]Mass of the solvent
\[\Delta T=\]Depression in freezing point of the solvent (determined experimentally)
(iii) Chemical methods
(a) Silver salt method for acids : It is based on the fact that silver salt of an organic acid on heating gives residue of metallic silver.
\[\underset{\text{Silver salt}}{\mathop{RCOOAg}}\,\xrightarrow{heat}\underset{\text{Silver (residue)}}{\mathop{Ag}}\,\]
From the mass of the silver salt taken and the mass of the silver residue obtained, the equivalent mass of the silver salt can be calculated.
\[\frac{\text{Equivalent mass of silver salt}}{\text{Equivalent mass of silver}}=\frac{\text{Mass of silver salt}}{\text{Mass of silver}}\]
Knowing the equivalent mass of silver salt, the equivalent mass of the acid can be obtained. The molecular mass of an acid can be determined with the help of the following relationship,
Mol. mass of the acid = Equivalent mass of the acid \[\times \] basicity
Calculations : (i) Mass of silver salt taken \[=wg\] (ii) Mass of metallic silver \[=x\,g\]
\[\frac{\text{Eq}\text{. mass of silver salt}}{\text{Eq}\text{. mass of silver }}=\frac{w}{x}\]; Eq. mass of silver salt \[=\frac{w}{x}\times 108\]
Let the equivalent mass of the acid be E. In the preparation of silver salt, a hydrogen atom of the carboxylic group is replaced by a silver atom.
Thus, Equivalent mass of silver salt \[=E-1+108\] \[=E+107\]
Thus, \[E+107=\frac{w}{x}\times 108\]or \[E=\left[ \frac{w}{x}\times 108-107 \right]\]
If n be the basicity of the acid, then Mol. Mass of the acid \[=\left[ \frac{w}{x}\times 108-107 \right]\times n\]
(b) Platinichloride method for bases : Organic bases combine with chloroplatinic acid, \[{{H}_{2}}PtC{{l}_{6}}\] to form insoluble platinichlorides, which, on ignition, leave a residue of metallic platinum. Knowing the mass of platinum salt and the mass of metallic platinum, the molecular mass of the platinum salt can be determined. Let B represents one molecule of the base. If the base is mono-acidic, the formula of the salt will be \[{{B}_{2}}{{H}_{2}}PtC{{l}_{6}}\].
\[{{B}_{2}}{{H}_{2}}PtC{{l}_{6}}\xrightarrow{heat}Pt\]
\[\frac{\text{Molecular mass of the salt}}{\text{Atomic mass of platinum }}=\frac{\text{Mass of platinum salt}}{\text{Mass of platinum}}\]
Let E be the equivalent mass of the base.
Molecular mass of the salt
\[=2E+2+195+213=2E+410\]
So \[\frac{2E+410}{195}=\frac{w}{x}=\frac{\text{Mass of platinum salt}}{\text{Mass of platinum}}\];
\[2E=\left[ \frac{w}{x}\times 195-410 \right]\] ; \[E=\frac{1}{2}\left[ \frac{w}{x}\times 195-410 \right]\]
Mol. mass of the base \[=\] Eq. mass \[\times \] acidity \[=E\times n\]
where n is the acidity of the base.
(c) Volumetric method for acids and bases : Molecular mass of an acid can be determined by dissolving a known mass of the acid in water and titrating the solution against a standard solution of an alkali using phenolphthalein as an indicator. Knowing the volume of alkali solution used, the mass of the acid, which will require 1000 ml of a normal alkali solution for complete neutralisation can be calculated. This mass of the acid will be its equivalent mass.
\[\underbrace{1000ml\,\,1N\,\,alkali\,\,solution}_{One\,\,gram\,\,equivalent\,\,of\,\,alkali}=\]One gram equivalent of the acid
Calculations : Suppose w g of the organic acid requires V ml N1 alkali solution for complete neutralisation.
V ml N1 alkali solution \[\equiv w\,gm\] acid
So 1000 ml N1 alkali solution \[\equiv \frac{w}{V\times {{N}_{1}}}\times 1000\,g\] acid \[\equiv \] one gram equivalent acid
Equivalent mass of the acid \[\equiv \frac{w}{V\times {{N}_{1}}}\times 1000\]
Thus, Molecular mass of the acid = Eq. mass \[\times \] basicity
In the case of organic bases, the known mass of the base is titrated against a standard solution of an acid. Knowing the volume of the acid solution used, the mass of the organic base which will require 1000 ml of a normal acid solution for complete neutralisation can be calculated. This mass will be the equivalent mass of the base.
\[\underbrace{1000ml\,\,1N\,\,acid\,\,solution}_{One\,\,gram\,\,equivalent\,\,of\,\,the\,\,acid}=\]One gram equivalent of the base
Molecular mass of the base \[=\] Eq. mass \[\times \] acidity
(5) Calculation of Empirical and Molecular formula
(i) Empirical formula : Empirical formula of a substance gives the simplest whole number ratio between the atoms of the various elements present in one molecule of the substance. For example, empirical formula of glucose is \[C{{H}_{2}}O\], i.e. for each carbon atom, there are two H-atoms and one oxygen atom. Its molecular formula is however, \[{{C}_{6}}{{H}_{12}}{{O}_{6}}\].
Calculation of empirical formula : The steps involved in the calculation are as follows,
(a) Divide the percentage of each element by its atomic mass. This gives the relative number of atoms.
(b) Divide the figures obtained in step (i) by the lowest one. This gives the simplest ratio of the various elements present.
(c) If the simplest ratio obtained in step (ii) is not a whole number ratio, then multiply all the figures with a suitable integer i.e., 2, 3, etc. to make it simplest whole number ratio.
(d) Write down the symbols of the various elements side by side with the above numbers at the lower right corner of each. This gives the empirical or the simplest formula.
(ii) Molecular formula : Molecular formula of a substance gives the actual number of atoms present in one molecule of the substance.
Molecular formula = \[n\times \]Empirical formula
Where, n is a simple integer 1, 2, 3,...... etc. given by the equation,
\[n=\frac{\text{Molecular mass of the compound}}{\text{Empirical formula mass of the compound}}\]
where the molecular mass of the compound is determined experimentally by any one of the methods discussed former, empirical formula mass is calculated by adding the atomic masses of all the atoms present in the empirical formula.
(iii) Molecular formula of gaseous hydrocarbons (Eudiometry)
Eudiometry is a direct method for determination of molecular formula of gaseous hydrocarbons without determining the percentage composition of various elements in it and without knowing the molecular weight of the hydrocarbon. The actual method used involves the following steps,
(a) A known volume of the gaseous hydrocarbon is mixed with an excess (known or unknown volume) of oxygen in the eudiometer tube kept in a trough of mercury.
(b) The mixture is exploded by passing an electric spark between the platinum electrodes. As a result, carbon and hydrogen of the hydrocarbon are oxidised to \[C{{O}_{2}}\]and \[{{H}_{2}}O\] vapours respectively.
(c) The tube is allowed to cool to room temperature when water vapours condense to give liquid water which has a negligible volume as compared to the volume of water vapours, Thus, the gaseous mixture left behind in the eudiometer tube after explosion and cooling consists of only \[C{{O}_{2}}\] and unused \[{{O}_{2}}\].
(d) Caustic potash or caustic soda solution is then introduced into the eudiometer tube which absorbs \[C{{O}_{2}}\] completely and only unused \[{{O}_{2}}\] is left behind. \[2NaOH+C{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\]
Thus, the decrease in volume on introducing NaOH or KOH solution gives the volume of \[C{{O}_{2}}\] formed. Sometimes, the volume of \[{{O}_{2}}\] left unused is found by introducing pyrogallol and noting the decrease in volume.
Calculation : From the volume of \[C{{O}_{2}}\] formed and the total volume of \[{{O}_{2}}\] used, it is possible to calculate the molecular formula of gaseous hydrocarbon with the help of the following equation.
\[\underset{1\,\,vol}{\mathop{{{C}_{x}}{{H}_{y}}}}\,+\underset{(x+y/4)vol}{\mathop{(x+y){{O}_{2}}}}\,\xrightarrow[{}]{{}}\underset{x\,\,vol}{\mathop{xC{{O}_{2}}}}\,+\underset{y/2\,\,vol}{\mathop{y/2{{H}_{2}}O}}\,\]
(Negligible volume on condensation)
From the above equation, it is evident that for one volume of hydrocarbon,
(a) \[(x+y/4)\] volume of \[{{O}_{2}}\] is used
(b) x volume of \[C{{O}_{2}}\] is produced
(c) y/2 volume of \[{{H}_{2}}O\] vapours is produced which condense to give liquid \[{{H}_{2}}O\] with negligible volume.
(d) Contraction on explosion and cooling
\[=[(1+x+y/4)-x]=1+y/4\]
By equating the experimental values with the theoretical values from the above combustion equation, the values of x and y and hence the molecular formula of the gaseous hydrocarbon can be easily determined.
(6) Determination of structure by spectroscopic and diffraction methods : The structures of organic substances are determined by spectroscopic and diffraction methods.
You need to login to perform this action.
You will be redirected in
3 sec
