Introduction
Category : JEE Main & Advanced
Carbonyl compounds are of two types, aldehydes and ketones. Both have a carbon-oxygen double bond often called as carbonyl group.

Both aldehyde and ketones possess the same general formula \[{{C}_{n}}{{H}_{2n}}O\].
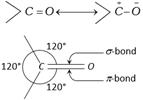
Structure : Carbonyl carbon atom is joined to three atoms by sigma bonds. Since these bonds utilise \[s{{p}^{2}}\]-orbitals, they lie in the same plane and are 120° apart. The carbon-oxygen double bond is different than carbon-carbon double bond. Since, oxygen is more electronegative, the electrons of the bond are attracted towards oxygen. Consequently, oxygen attains a partial negative charge and carbon a partial positive charge making the bond polar. The high values of dipole moment, ![]()
(2.3 - 2.8D) cannot be explained only on the basis of inductive effect and thus, it is proposed that carbonyl group is a resonance hybrid of the following two structures.
You need to login to perform this action.
You will be redirected in
3 sec
