Acetophenone, \[{{\mathbf{C}}_{\mathbf{6}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{COC}{{\mathbf{H}}_{\mathbf{3}}}\mathbf{,}\] Acetyl Benzene
Category : JEE Main & Advanced
(1) Method of preparation
(i) Friedel-Craft's reaction : Acetyl chloride reacts with benzene in presence of anhydrous aluminium chloride to form acetophenone.
![]()
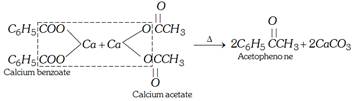
(ii) By distillation of a mixture of calcium benzoate and calcium acetate.
(iii) By methylation of benzaldehyde with diazomethane.
\[{{C}_{6}}{{H}_{5}}CHO+C{{H}_{2}}{{N}_{2}}\xrightarrow{{}}{{C}_{6}}{{H}_{5}}COC{{H}_{3}}+{{N}_{2}}\]
(iv) By treating benzoyl chloride with dimethyl cadmium.
\[2{{C}_{6}}{{H}_{5}}COCl+{{(C{{H}_{3}})}_{2}}Cd\xrightarrow{{}}2{{C}_{6}}{{H}_{5}}COC{{H}_{3}}+CdC{{l}_{2}}\]
(v) By Grignard reagent
(a) \[C{{H}_{3}}C\equiv N+{{C}_{6}}{{H}_{5}}MgBr\xrightarrow{{}}\,C{{H}_{3}}\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{C}}\,\,\,\,\,=\,\,\,}}\,NMgBr\xrightarrow[{}]{{{H}_{2}}O}\]\[{{C}_{6}}{{H}_{5}}COC{{H}_{3}}+N{{H}_{3}}+Mg(OH)Br\]
(b) 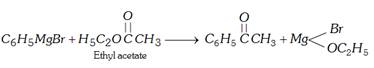
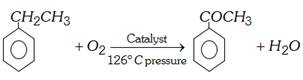
(vi) Commercial preparation : Ethylbenzene is oxidised with air at \[{{126}^{o}}C\] under pressure in presence of a catalyst manganese acetate.
(2) Physical properties : It is a colourless crystalline solid with melting point \[{{20}^{o}}C\] and boiling point \[{{202}^{o}}C\]. It has characteristic pleasant odour. It is slightly soluble in water. Chemically, It is similar to acetone.
(3) Chemical properties :
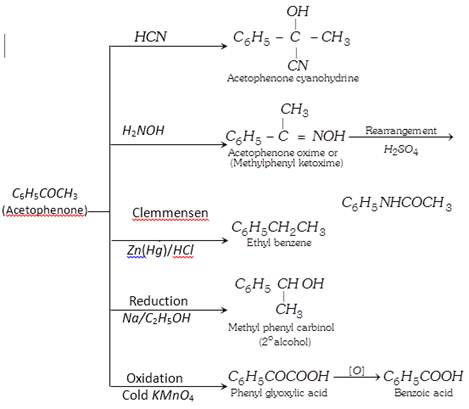
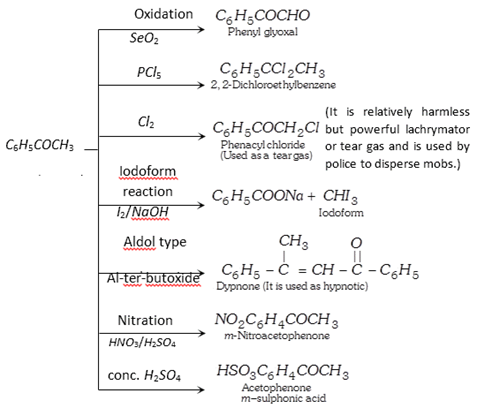
(4) Uses : It is used in perfumery and as a sleep producing drug.
You need to login to perform this action.
You will be redirected in
3 sec
