Higher Fatty Acids
Category : JEE Main & Advanced
Palmitic, stearic and oleic acids are found in natural fats and oils as glyceryl esters.
They have derived their names from the natural source from which they are prepared by hydrolysis with alkali.
| Name of acids | Source | Molecular formula |
| Palmitic acid | Palm oil | \[C{{H}_{3}}{{(C{{H}_{2}})}_{14}}COOH\] |
| Stearic acid | Stear (meaning tallow) | \[C{{H}_{3}}{{(C{{H}_{2}})}_{16}}COOH\] |
| Oleic acid | Olive oil. | \[C{{H}_{3}}{{(C{{H}_{2}})}_{7}}CH=CH{{(C{{H}_{2}})}_{7}}COOH\] |
Palmitic\[\]and stearic acids are waxy colourless solids with melting points \[{{64}^{o}}C\] and \[{{72}^{o}}C,\] respectively. They are insoluble in water but soluble in ethanol and ether. They find use in the manufacture of soaps and candles. Soaps contain sodium or potassium salts of these higher fatty acids.
Oleic acid has low melting point, i.e., \[{{16}^{o}}C\]. It is insoluble in water but soluble in alcohol and ether. Besides the reactions of acids, it also gives reactions of alkenes. Two aldehydes are formed on ozonolysis.
\[C{{H}_{3}}{{(C{{H}_{2}})}_{7}}CH=CH{{(C{{H}_{2}})}_{7}}COOH\underset{(ii)Zn+{{H}_{2}}O}{\mathop{\xrightarrow{(i){{O}_{3}}}}}\,\] \[C{{H}_{3}}{{(C{{H}_{2}})}_{7}}CHO+HOOC{{(C{{H}_{2}})}_{7}}CHO\]
It is used for making soaps, lubricants and detergents.
(1) Difference between oils and fats : Oils and fats belong to the same chemical group, yet they are different in their physical state.
(i) Oils are liquids at ordinary temperature (below \[{{20}^{o}}C\]) while fats are semi solids or solids (their melting points are more than \[{{20}^{o}}C\]). A substance may be classed as fat in one season and oil in another season or the same glyceride may be solid at a hill station and liquid in plains. Thus, this distinction is not well founded as the physical state depends on climate and weather.
(ii) The difference in oils and fats is actually dependent on the nature of monocarboxylic acid present in the glyceride. Oils contain large proportion of the glycerides of lower carboxylic acids, (e.g., butyric acid, caprylic acid and caproic acid) and unsaturated fatty acids, (e.g., oleic, linoleic and linolenic acids) while fats contain a large proportion of glycerides of higher saturated carboxylic acids, (e.g., palmitic, stearic acids).
Lard (fat of hogs) is a solid fat and its composition in terms of fatty acids produced on hydrolysis is approximately 32% palmitic acid, 18% stearic acid, 45% oleic acid and 5% linolenic acid. Olive oil on the other hand, contains 84% oleic acid, 4% linoleic acid, 9% palmitic acid and 3% stearic acid.
(2) Physical Properties of oils and Fats
(i) Fats are solids, whereas oils are liquids.
(ii) They are insoluble in water but soluble in ether, chloroform and benzene.
(iii) They have less specific gravity than water and consequently float on the surface when mixed with it.
(iv) Pure fats and oils are colourless, odourless and tasteless but natural fats and oils possess a characteristic odour due to presence of other substances.
(v) They have specific melting points, specific gravity and refractive index hence they can be identified by these oil constants.
(vi) Animal fats contain cholesterol, an unsaturated alcohol, whereas vegetable fats contains phytosterol.
(3) Chemical Properties : They give reactions of carbon-carbon double bonds and ester groups.
(i) Hydrolysis
(a) By superheated steam
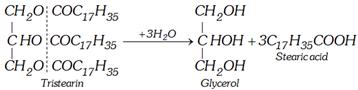
(b) Base hydrolysis [Saponification]
\[\underset{\text{Fat or oil}}{\mathop{\underset{C{{H}_{2}}OCOR}{\overset{C{{H}_{2}}OCOR}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOCOR}}}\,}}\,+3NaOH\to \underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH}}}\,}}\,\underset{\begin{smallmatrix} \text{Sa}\text{lt fatty acid} \\ \text{ (Soap)} \end{smallmatrix}}{\mathop{+3RCOONa}}\,\]
(c) Enzyme hydrolysis : Enzyme like lipase, when added to an emulsion of fat in water, hydrolyses it into acid and glycerol in about two or three days.
(ii) Hydrogenation : In the presence of finally divided nickel, at low pressure the hydrogenation process is called hardening of oils.
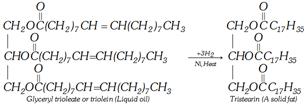
(iii) Hydrogenolysis [Reduction to alcohol]
\[\underset{\underset{\underset{\text{Tristearin}}{\mathop{C{{H}_{2}}-O-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{17}}{{H}_{35}}}}\,\ \ \ \ }{\mathop{CH-O-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{17}}{{H}_{35}}}}\,\underset{200\,atm}{\mathop{\xrightarrow{6{{H}_{2}}}}}\,\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH\ }}}\,+\ \underset{\text{Octadecyl alcohol}}{\mathop{3{{C}_{17}}{{H}_{35}}C{{H}_{2}}OH}}\,}{\mathop{C{{H}_{2}}-O-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-{{C}_{17}}{{H}_{35}}\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ }}\,\]
(iv) Drying : Certain oils, containing glycerides of unsaturated fatty acids having two or three double bonds have the tendency of slowly absorbing oxygen from atmosphere and undergoing polymerisation to form hard transparent coating. This process is known as drying and such oils are called drying oils. Unsaturated oils such as linseed oil are, therefore, used as medium of paints and varnishes.
(v) Rancidification : On long storage in contact with air and moisture, oils and fats develop unpleasant smell. The process is known as rancidification. It is believed that rancidification occurs due to hydrolysis-oxidation.
(4) Analysis of oils and fats
(i) Acid value : It indicates the amount of free acid present in the oil or fat. It is defined as the number of milligrams of KOH required to neutralize the free acid present in one gram of the oil or fat. It is determined by dissolving a weighed amount of oil or fat in alcohol and titrating it against a standard solution of KOH using phenolphthalein as an indicator.
(ii) Saponification value : It is a measure of fatty acids present as esters in oils and fats. It is defined as the number of milligrams of KOH required to saponify one gram of the oil or fat or number of milligrams of KOH required to neutralize the free acids resulting from the hydrolysis of one gram of an oil or fat. It is determined by refluxing a Saponification number of fat or oil
\[=\frac{168,000}{M},\] Where M = molecular mass
(iii) Iodine value : Iodine value of a fat or oil is a measure of its degree of unsaturation. It is defined as the number of grams of iodine taken up by 100 grams of fat or oil for saturation. For a saturated acid glyceride, the iodine value is zero. Thus, the iodine value for a fat is low whereas for oil, it is high. As iodine does not react readily, in actual practice, iodine monochloride is used. Iodine monochloride is known as Wij's reagent.
(iv) Reichert-Meissl value, (R/M value) : It indicates the amount of steam volatile fatty acids present in the oil or fat. It is defined as the number of millilitres of 0.1 N KOH solution required to neutralize the distillate of 5 grams of hydrolysed fat. It is determined by hydrolysing a known weighed amount (5 grams) of the fat with alkali solution and the mixture is acidified with dilute sulphuric acid and steam distilled. The distillate is cooled, filtered and titrated against 0.1 N KOH.
(5) Uses
(i) Many oils and fats are used as food material.
(ii) Oils and fats are used for the manufacture of glycerol, fatty acids, soaps, candles, vegetable ghee, margarine, hair oils, etc.
(iii) Oils like linseed oil, tung oil, etc., are used for the manufacture of paints, varnish, etc.
(iv) Castor oil is used as purgative and codliver oil as a source of vitamins A and D. Almond oil is used in pharmacy. Olive oil is also used as medicine.
(v) Oils are also used as lubricants and illuminants.
Difference between vegetable oils and Mineral oils
| Property | Vegetable oils | Minerals oils |
| 1. Composition | These are triesters of glycerol with higher fatty acids. | These are hydrocarbons (saturated). Kerosene oil?Alkanes from \[{{C}_{12}}\] to \[{{C}_{16}}\]. |
| 2. Source | Seeds root and fruits of plants. | These occur inside earth in the form of petroleum. |
| 3. Hydrolysis | Undergo hydrolysis with alkali. Form soap and glycerol. | No hydrolysis occurs. |
| 4. On adding NaOH and phenolphthalein | Decolourisation of pink colour occurs. | No effect. |
| 5. Burning | Burns slowly | Burn very readily. |
| 6. Hydrogenation | Hydrogenation occurs in presence of nickel catalyst. Solid glycerides (fats) are formed. | No hydrogenation occurs.. |
(6) Soaps : Soaps are the metallic salts of higher fatty acids such as palmitic, stearic, oleic, etc. The sodium and potassium salts are the common soaps which are soluble in water and used for cleansing purposes. Soaps of other metals such as calcium, magnesium, zinc, chromium, lead, etc., are insoluble in water. These are not used for cleansing purposes but for other purposes (lubricants, driers, adhesives, etc.)
Ordinary soaps (sodium and potassium) are the products of hydrolysis of oils and fats with sodium hydroxide or potassium hydroxide. The oils and fats are mixed glycerides and thus soaps are mixtures of salts of saturated and unsaturated long chain carboxylic acids containing 12 to 18 carbon atoms. This process always yields glycerol as a byproduct.
\[\underset{\text{Triglyceride}}{\mathop{\underset{C{{H}_{2}}OCO{{R}_{3}}}{\overset{C{{H}_{2}}OCO{{R}_{1}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOCO{{R}_{2}}}}}\,}}\,\,\,+\,\,3NaOH\to \underset{\text{Glycerol}}{\mathop{\underset{C{{H}_{2}}OH}{\overset{C{{H}_{2}}OH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,HOH}}}\,}}\,\,\,+\,\,\underset{\text{Soap}}{\mathop{\underset{{{R}_{3}}COONa}{\overset{{{R}_{1}}COONa}{\mathop{\underset{+}{\overset{+}{\mathop{{{R}_{2}}COONa}}}\,}}}\,}}\,\]
There are three methods for manufacture of soaps :
(i) The cold process
(ii) The hot process
(iii) Modern process
(7) Synthetic Detergents : The synthetic detergents or Syndets are substitutes of soaps. They have cleansing power as good or better than ordinary soaps. Like soap, they contain both hydrophilic (water soluble) and hydrophobic (oil-soluble) parts in the molecule.

Some of the detergents used these days are given below:
(i) Sodium alkyl sulphates : These are sodium salts of sulphuric acid esters of long chain aliphatic alcohols containing usually 10 to 15 carbon atoms. The alcohols are obtained from oils or fats by hydrogenolysis.
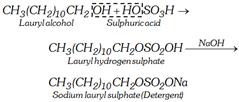
The other examples are sodium cetyl sulphate, \[{{C}_{16}}{{H}_{33}}OS{{O}_{2}}ONa\] and sodium stearyl sulphate, \[C{{H}_{3}}{{(C{{H}_{2}})}_{16}}C{{H}_{2}}OS{{O}_{3}}Na\]. Unlike ordinary soaps, they do not produce OH– ions on hydrolysis and thus can be safely used for woollen garments.
(ii) Sodium alkyl benzene sulphonates : Sodium p-dodecyl benzene sulphonate (S.D.S.) acts as a good detergent. It is most widely used since 1975.
\[\underset{1\text{-Dodecene}}{\mathop{C{{H}_{3}}{{(C{{H}_{2}})}_{9}}CH=C{{H}_{2}}}}\,+{{C}_{6}}{{H}_{6}}\xrightarrow{AlC{{l}_{3}}}\]\[\underset{\text{2-Dodecyl benzene}}{\mathop{C{{H}_{3}}{{(C{{H}_{2}})}_{9}}\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,H}}\,-}}\,{{C}_{6}}{{H}_{5}}\]\[\underset{(ii)NaOH}{\mathop{\xrightarrow{(i){{H}_{2}}S{{O}_{4}}}}}\,\] \[\underset{\text{(S}\text{.D}\text{.S}\text{.)}}{\mathop{C{{H}_{3}}-{{(C{{H}_{2}})}_{9}}-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,H}}\,-{{C}_{6}}{{H}_{4}}-S{{O}_{3}}Na}}\,\]
These long chain alkyl benzene sulphonate (L.A.S.) are most widely used syndets.
(iii) Quaternary ammonium salts : Quaternary ammonium salts with long chain alkyl group have been used as detergents, e.g., trimethyl stearyl ammonium bromide.
![]()
(iv) Sulphonates with triethanol ammonium ion in place of sodium serve as highly soluble materials for liquid detergents.
![]()
(v) Partially esterified polyhydroxy compounds also acts as detergents.
\[\underset{\text{Pentaerythritol monostearate}}{\mathop{{{C}_{17}}{{H}_{35}}COOC{{H}_{2}}-\underset{C{{H}_{2}}OH\,\,\,}{\overset{C{{H}_{2}}OH\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{2}}O}}}\,H}}\,\]
Detergents are superior cleansing agents due to following properties.
(i) These can be used both in soft and hard waters as the calcium and magnesium ions present in hard water form soluble salts with detergents. Ordinary soap cannot be used in hard water.
(ii) The aqueous solution of detergents are neutral. Hence these can be used for washing all types of fabrics without any damage. The solution or ordinary soap is alkaline and thus cannot be used to wash delicate fabrics.
(8) Waxes : Waxes are the esters of higher fatty acids with higher monohydric alcohols. The acids and alcohols commonly found in waxes are palmitic, cerotic acid \[({{C}_{25}}{{H}_{51}}COOH)\], melissic acid \[({{C}_{30}}{{H}_{61}}COOH)\] and cetyl alcohol \[({{C}_{16}}{{H}_{33}}OH)\], ceryl alcohol \[({{C}_{26}}{{H}_{53}}OH)\], myricyl alcohol \[({{C}_{30}}{{H}_{61}}OH)\], etc.
Waxes are insoluble in water but are readily soluble in benzene, petroleum, carbon disulphide etc. Waxes on hydrolysis with water yields higher fatty acids and higher monohydric alcohols.
\[\underset{\text{Cetyl palmitate}}{\mathop{{{C}_{15}}{{H}_{31}}COO{{C}_{16}}{{H}_{33}}}}\,+{{H}_{2}}O\to \underset{\text{Palmitic acid}}{\mathop{{{C}_{15}}{{H}_{31}}COOH}}\,+\underset{\text{Cetyl alcohol}}{\mathop{{{C}_{16}}{{H}_{33}}OH}}\,\]
When hydrolysis is carried with caustic alkalies, soap and higher monohydric alcohols are formed.
\[{{C}_{15}}{{H}_{31}}COO{{C}_{16}}{{H}_{33}}+NaOH\to {{C}_{16}}{{H}_{33}}OH+\underset{\text{Sodium palmitate (Soap)}}{\mathop{{{C}_{15}}{{H}_{31}}COONa}}\,\]
The common waxes are:
(i) Bees wax, Myricyl palmitate, \[{{C}_{15}}{{H}_{31}}COO{{C}_{30}}{{H}_{61}}\]
(ii) Spermaceti wax, Cetyl palmitate, \[{{C}_{15}}{{H}_{31}}COO{{C}_{16}}{{H}_{33}}\]
(iii) Carnauba wax, Myricyl cerotate, \[{{C}_{25}}{{H}_{51}}COO{{C}_{30}}{{H}_{61}}\]
Waxes are used in the manufacture of candles, polishes, inks, water proof coating and cosmetic preparations.
Waxes obtained from plants and animals are different than paraffin wax which is a petroleum product and a mixture of higher hydrocarbons (20 to 30 carbon atoms). So paraffin wax is not an ester.
Candles are prepared by mixing paraffin wax (90%) with higher fatty acids like stearic and palmitic. The fatty acids are added to paraffin wax as to give strength to candles. The mixture is melted and poured into metal tubes containing streched threads. On cooling candles are obtained.
You need to login to perform this action.
You will be redirected in
3 sec
