Aromatic Carbonyl Compounds
Category : JEE Main & Advanced
Aromatic aldehydes are of two types :
The compounds in which \[-CHO\] group is attached directly to an aromatic ring, e.g., benzaldehyde, \[{{C}_{6}}{{H}_{5}}CHO\].
Those in which aldehyde \[(-CHO)\] group is attached to side chain, e.g., phenyl acetaldehyde, \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}CHO\]. They closely resemble with aliphatic aldehydes.
Aromatic ketones are compounds in which a carbonyl group \[(\,>\,C=O)\] is attached to either two aryl groups or one aryl group and one alkyl group. Examples are :
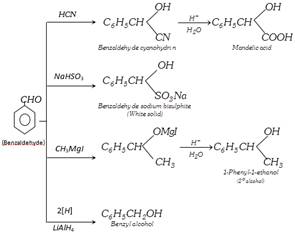
Benzaldehyde is the simplest aromatic aldehyde. It occurs in bitter almonds in the form of its glucoside, amygdalin \[({{C}_{20}}{{H}_{27}}{{O}_{11}}N)\]. When amygdalin is boiled with dilute acids, it hydrolyses into benzaldehyde, glucose and HCN
\[\underset{\text{Amygdalin}}{\mathop{\overset{CN\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}CHO{{C}_{12}}{{H}_{21}}{{O}_{10}}}}\,}}\,}}\,+2{{H}_{2}}O\xrightarrow{{}}\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,+\] \[\underset{\text{Glucose}}{\mathop{2{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+HCN\]
Benzaldehyde is also known as oil of bitter almonds.
(1) Method of preparation
(i) Laboratory method : It is conveniently prepared by boiling benzyl chloride with copper nitrate or lead nitrate solution in a current of carbon dioxide.
\[\underset{\text{Benzyl chloride}}{\mathop{2{{C}_{6}}{{H}_{5}}C{{H}_{2}}Cl}}\,+\underset{Pb{{(N{{O}_{3}})}_{2}}}{\mathop{\underset{\text{or}}{\mathop{Cu{{(N{{O}_{3}})}_{2}}}}\,}}\,\underset{C{{O}_{2}}}{\mathop{\xrightarrow{\text{heat}}}}\,\underset{\text{Benzaldehyde}}{\mathop{2{{C}_{6}}{{H}_{5}}CHO}}\,+CuC{{l}_{2}}+2HN{{O}_{2}}\]
\[[2HN{{O}_{2}}\xrightarrow{{}}NO+N{{O}_{2}}+{{H}_{2}}O]\]
(ii) Rosenmund reaction :
\[\underset{\text{Benzyl chloride}}{\mathop{{{C}_{6}}{{H}_{5}}COCl+{{H}_{2}}}}\,\underset{\text{xylene}}{\mathop{\xrightarrow{Pd/BaS{{O}_{4}}}}}\,\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,+HCl\]
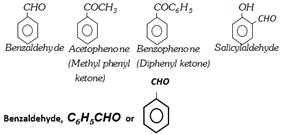
(iii) By dry distillation of a mixture of calcium benzoate and calcium formate
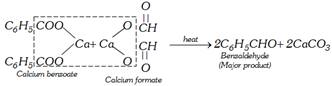
(iv) By oxidation of benzyl alcohol : This involves the treatment of benzyl alcohol with dil. \[HN{{O}_{3}}\] or acidic potassium dichromate or chromic anhydride in acetic anhydride or with copper catalyst at \[{{350}^{o}}C\].
This method is used for commercial production of benzaldehyde.
(v) By hydrolysis of benzal chloride :
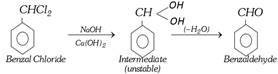
This is also an industrial method.
(vi) By oxidation of Toluene
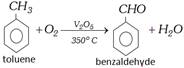
Commercially the oxidation of toluene is done with air and diluted with nitrogen (to prevent complete oxidation) at \[{{500}^{o}}C\] in the presence of oxides of \[Mn,\,Mo\] or \[Zr\] as catalyst.
Partial oxidation of toluene with manganese dioxide and dilute sulphuric acid at \[{{35}^{o}}C\], also forms benzaldehyde.
\[\underset{\text{Toluene}}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{3}}}}\,\underset{{{(C{{H}_{3}}CO)}_{2}}O}{\mathop{\xrightarrow{Cr{{O}_{3}}}}}\,\underset{\text{Benzyliden}\text{e acetate}}{\mathop{{{C}_{6}}{{H}_{5}}CH{{(OCOC{{H}_{3}})}_{2}}}}\,\xrightarrow{{{H}^{+}}/{{H}_{2}}O}\]
\[{{C}_{6}}{{H}_{5}}CHO+2C{{H}_{3}}COOH\]
(vii) Etard's reaction :
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}+2Cr{{O}_{2}}C{{l}_{2}}\xrightarrow{{}}\]
\[\underset{\text{Brown addition product}}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{3}}2Cr{{O}_{2}}C{{l}_{2}}}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,\]
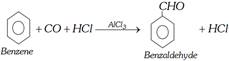
(viii) Gattermann-koch aldehyde synthesis : Benzene is converted into benzaldehyde by passing a mixture of carbon monoxide and HCl gas under high pressure into the ether solution of benzene in presence of anhydrous aluminium chloride and cuprous chloride.
(ix) Gattermann reaction
\[HC\equiv N+HCl+AlC{{l}_{3}}\xrightarrow{{}}H\overset{\oplus }{\mathop{C}}\,=NH+AlCl_{4}^{-}\];
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{5}}H}}\,+\overset{\,\,\,\,\,\,+}{\mathop{HC}}\,=NH\xrightarrow{{}}{{C}_{6}}{{H}_{5}}CH=\overset{+\,\,\,\,\,\,\,}{\mathop{N{{H}_{2}}}}\,\]
\[{{C}_{6}}{{H}_{5}}CH=\overset{+\,\,\,\,\,\,}{\mathop{N{{H}_{2}}}}\,+{{H}_{2}}O+AlCl_{4}^{-}\xrightarrow{{}}\]
\[{{C}_{6}}{{H}_{5}}CHO+N{{H}_{3}}+AlC{{l}_{3}}+HCl\]
Thus,
![]()
(x) Stephen's reaction : Benzaldehyde is obtained by partial reduction of phenyl cyanide with stannous chloride and passing dry \[HCl\] gas in ether solution followed by hydrolysis of the aldimine stannic chloride with water.
\[\underset{\text{Phenyl cyanide}}{\mathop{{{C}_{6}}{{H}_{5}}C\equiv N}}\,\underset{\text{Ether}}{\mathop{\xrightarrow{HCl/SnC{{l}_{2}}}}}\,\underset{\text{Aldimine complex}}{\mathop{{{[{{C}_{6}}{{H}_{5}}CH=NH]}_{2}}{{H}_{2}}SnC{{l}_{6}}}}\,\]
\[\xrightarrow{{{H}_{2}}O}2{{C}_{6}}{{H}_{5}}CHO\]
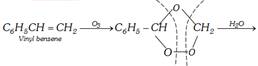
(xi) By ozonolysis of styrene
(xii) Grignard reaction
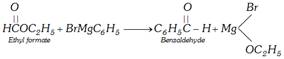
Other reagents like carbon monoxide or HCN can also be used in place of ethyl formate.
(xiii) From Diazonium salt

(2) Physical properties
(i) Benzaldehyde is a colourless oily liquid. Its boiling point is \[{{179}^{o}}C\].
(ii) It has smell of bitter almonds.
(iii) It is sparingly soluble in water but highly soluble in organic solvents.
(iv) It is steam volatile.
(v) It is heavier than water (sp. gr. 1.0504 at \[{{15}^{o}}C\]).
(vi) It is poisonous in nature.
(3) Chemical properties
(i) Addition reaction: The carbonyl group is polar as oxygen is more electronegative than carbon,
![]()
Thus, The positive part of the polar reagent always goes to the carbonyl oxygen and negative part goes to carbonyl carbon.
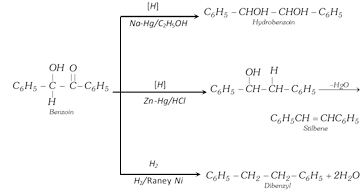
However on reduction with sodium amalgam and water, it gives hydrobenzoin,
\[{{C}_{6}}{{H}_{5}}CH=O+2H+O=CH{{C}_{6}}{{H}_{5}}\underset{{{H}_{2}}O}{\mathop{\xrightarrow{Na-Hg}}}\,\]
\[\underset{\text{Hydrobenzo}\text{in}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,OH}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{{{C}_{6}}{{H}_{5}}CH}}\,}}\,-\underset{OH}{\mathop{\underset{\,|\,\,\,\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-}}\,{{C}_{6}}{{H}_{5}}\]
(ii) Reactions involving replacement of carbonyl oxygen
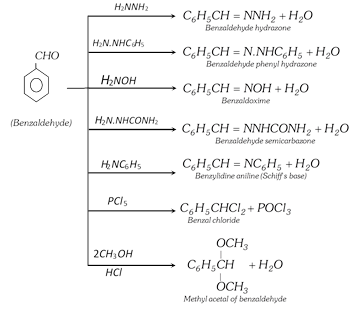
(iii) Oxidation : Benzaldehyde is readily oxidised to benzoic acid even on exposure to air.
\[{{C}_{6}}{{H}_{5}}CHO\xrightarrow{[O]}{{C}_{6}}{{H}_{5}}COOH\]
Acidified \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\], alkaline \[KMn{{O}_{4}}\] and dilute \[HN{{O}_{3}}\] can be used as oxidising agents for oxidation.
(iv) Reducing properties : Benzaldehyde is a weak reducing agent. It reduces ammonical silver nitrate solution (Tollen's reagent) to give silver mirror but does not reduce Fehling's solution.
\[\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,+A{{g}_{2}}O\xrightarrow{{}}2Ag+\underset{\text{Benzoic acid}}{\mathop{{{C}_{6}}{{H}_{5}}COOH}}\,\]
(v) Clemmensen's reduction : With amalgamated zinc and conc. HCl, benzaldehyde is reduced to toluene.
\[{{C}_{6}}{{H}_{5}}CHO+4H\underset{HCl}{\mathop{\xrightarrow{Zn-Hg}}}\,{{C}_{6}}{{H}_{5}}C{{H}_{3}}+{{H}_{2}}O\]
(vi) Schiff's reaction: It restores pink colour to Schiff's reagent (aqueous solution of p-rosaniline hydrochloride decolourised by passing sulphur dioxide).
(vii) Tischenko reaction : On heating benzaldehyde with aluminium alkoxide (ethoxide) and a little of anhydrous \[AlC{{l}_{3}}\] or \[ZnC{{l}_{2}}\], it undergoes an intermolecular oxidation and reduction (like aliphatic aldehydes) to form acid and alcohol respectively as such and react to produce benzyl benzoate (an ester).
\[\underset{\text{Benzaldehyde}}{\mathop{2{{C}_{6}}{{H}_{5}}CHO}}\,\xrightarrow{Al{{(O{{C}_{2}}{{H}_{5}})}_{3}}}\underset{\text{Benzyl benzoate (ester)}}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}OOC{{C}_{6}}{{H}_{5}}}}\,\]
(viii) Reactions in which benzaldehyde differs from aliphatic aldehydes
(a) With fehling's solution : No reaction
(b) Action of chlorine : Benzoyl chloride is formed when chlorine is passed through benzaldehyde at its boiling point in absence of halogen carrier. This is because in benzaldehyde there is no \[\alpha \]-hydrogen atom present which could be replaced by chlorine.
\[{{C}_{6}}{{H}_{5}}CHO+C{{l}_{2}}\underset{\Delta }{\mathop{\xrightarrow{{{170}^{o}}C}}}\,{{C}_{6}}{{H}_{5}}COCl+HCl\]
(c) Cannizzaro's reaction :
\[\underset{\text{Benzaldehyde}}{\mathop{2{{C}_{6}}{{H}_{5}}CHO}}\,\xrightarrow{KOH}\]\[\underset{\text{Benzyl alcohol}}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH}}\,+\underset{\text{Potassium benzoate}}{\mathop{{{C}_{6}}{{H}_{5}}COOK}}\,\]
The possible Mechanism is
First step is the reversible addition of hydroxide ion to carbonyl group.
\[{{C}_{6}}{{H}_{5}}-\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,=O+O{{H}^{-}}\,\,\underset{\text{Anion (I)}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{OH}{\overset{H}{\mathop{\underset{|\,\,\,\,\,}{\overset{|\,\,}{\mathop{C\,\,}}}\,}}}\,-{{O}^{-}}}}\,\]
Second step is the transfer of hydride ion directly to the another aldehyde molecule, the latter is thus reduced to alkoxide ion and the former (ion I) is oxidised to an acid.
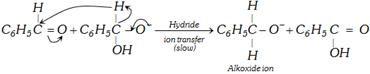
\[\underset{\text{Benzyl alcohol}}{\mathop{{{C}_{6}}{{H}_{5}}-\overset{\begin{matrix} +{{H}^{+}} \\ {} \\ \end{matrix}\,}{\mathop{\underset{\begin{smallmatrix} H \\ \end{smallmatrix}}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-}}\,\,OH}}\,+\underset{\text{Benzoate ion}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{{{O}^{-\,\,}}}{\mathop{\underset{|\,\,}{\mathop{C\,\,}}\,=}}\,O}}\,\]
Third Step is exchange of protons to give most stable pair alcohol and acid anion.
So one molecule of aldehyde acts as hydride donor and the other acts as hydride acceptor. In other words, Cannizzaro's reaction is an example of self reduction and oxidation.
\[\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO}}\,+\underset{\text{Formaldehyde}}{\mathop{HCHO}}\,\underset{\text{heat}}{\mathop{\xrightarrow{NaOH}}}\,\underset{\text{Benzyl alcohol}}{\mathop{{{C}_{6}}{{H}_{5}}C{{H}_{2}}OH}}\,+\underset{\text{Sod}\text{. formate}}{\mathop{HCOONa}}\,\]
Aldehyde which do not have \[\alpha \]- hydrogen (\[{{C}_{6}}{{H}_{5}}-CHO,CC{{l}_{3}}CHO,\,{{(C{{H}_{3}})}_{3}}C-CHO,\,C{{H}_{2}}O\] etc.) undergoes Cannizzaro’s reaction.
Intramolecular cannizzaro reaction
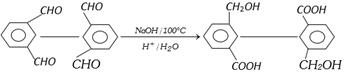
(d) Benzoin Condensation
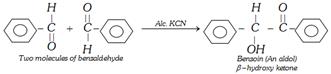
Benzoin can also be reduced to a number of product i.e.,
Benzoin can be readily oxidised to a diketone, i.e, benzil.
\[\underset{\text{Benzoin}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{OH\,}{\mathop{\underset{|\,\,\,\,\,\,}{\mathop{CH}}\,}}\,-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}}}\,+[O]\underset{{{H}_{2}}O}{\mathop{\underset{\text{Pyridine}}{\mathop{\xrightarrow{CuS{{O}_{4}}}}}\,}}\,\underset{\text{Benzil}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-{{C}_{6}}{{H}_{5}}}}\,\]
(e) Perkin's reaction
\[\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CH\,O}}\,+{{H}_{2}}\,\underset{\text{Acetic anhydride}}{\mathop{CHCOOCOC{{H}_{3}}}}\,\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{C{{H}_{3}}COONa}}}\,\] \[{{C}_{6}}{{H}_{5}}CH=CHCOOCOC{{H}_{3}}\] \[\xrightarrow{{{H}_{2}}O}\underset{\text{Cinnamic acid}}{\mathop{{{C}_{6}}{{H}_{5}}CH=CHCOOH}}\,+\underset{\text{Acetic acid}}{\mathop{C{{H}_{3}}COOH}}\,\]
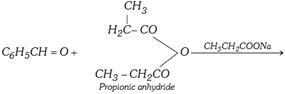
(f) Claisen condensation [Claisen-schmidt reaction]
\[\underset{\alpha \text{-Methyl cinnamic acid}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}CH=\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,\,\,\,-}}\,COOH+}}\,C{{H}_{3}}C{{H}_{2}}COONa\]
\[\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{Propionaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CHO\,\,+{{H}_{2}}\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C\,\,-\,\,}}\,}}\,CHO\,\,}}\,\underset{\text{(Dil}\text{.)}}{\mathop{\xrightarrow{NaOH}}}\,\]
\[\underset{\alpha \text{-Methyl cinnamic aldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CH\overset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,}{\mathop{=\ \overset{|\,\,\,\,}{\mathop{C}}\,\ -\ CHO\,}}\,+{{H}_{2}}O}}\,\]
\[{{C}_{6}}{{H}_{5}}CHO+\underset{\text{Acetone}}{\mathop{{{H}_{2}}CHCOC{{H}_{3}}}}\,\xrightarrow{NaOH(\text{Dil}\text{.})}\]
\[\underset{\text{Benzyliden}\text{e acetone}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}CH=CHCOC{{H}_{3}}}}\,+{{H}_{2}}O\]
(g) Knoevenagel reaction
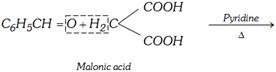
\[\underset{\text{Cinnamic acid}}{\mathop{{{C}_{6}}{{H}_{5}}CH=CHCOOH}}\,+C{{O}_{2}}+{{H}_{2}}O\]
(h) Reaction with aniline : Benzaldehyde reacts with aniline and forms Schiff's base
\[{{C}_{6}}{{H}_{5}}CH=O+\underset{\text{Aniline}}{\mathop{{{H}_{2}}N{{C}_{6}}{{H}_{5}}}}\,\underset{(-{{H}_{2}}O)}{\mathop{\xrightarrow{\text{Warm}}}}\,\underset{\text{(Schiff }\!\!'\!\!\text{ s base)}}{\mathop{\underset{\text{Benzylidene aniline}}{\mathop{{{C}_{6}}{{H}_{5}}CH=N{{C}_{6}}{{H}_{5}}}}\,}}\,\]
Reaction with Dimethylaniline
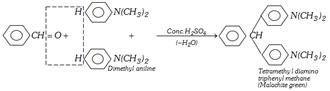
(i) Reaction with Ammonia : Benzaldehyde reacts with ammonia to form hydrobenzamide aldehyde other than \[C{{H}_{2}}O\] give aldehyde ammonia while \[C{{H}_{2}}O\] forms urotropine.
\[\begin{matrix}{{C}_{6}}{{H}_{5}}-CHO \\ {{C}_{6}}{{H}_{5}}-CHO \\\end{matrix}+\begin{matrix}{{H}_{2}}NH \\{{H}_{2}}NH \\\end{matrix}\xrightarrow{O=CH-{{C}_{6}}{{H}_{5}}}\]
![]()
(j) Reformatsky reaction
\[\underset{\text{Benzaldehyde}}{\mathop{{{C}_{6}}{{H}_{5}}CH=O}}\,+Zn+\underset{\text{Bromo ethylacetate}}{\mathop{Br\overset{\alpha }{\mathop{C}}\,{{H}_{2}}COO{{C}_{2}}{{H}_{5}}}}\,\xrightarrow{{}}\]
\[{{C}_{6}}{{H}_{5}}\underset{OZnBr\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CHC{{H}_{2}}COO{{C}_{2}}{{H}_{5}}}}\,}}\,\xrightarrow{{{H}_{2}}O}\underset{\beta \text{-hydroxy ester}}{\mathop{{{C}_{6}}{{H}_{5}}-\underset{OH}{\mathop{\underset{|\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}}}\,\]
(k) Reaction of benzene ring
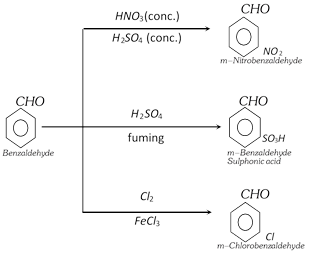
(4) Uses : Benzaldehyde is used,
(i) In perfumery
(ii) In manufacture of dyes
(iii) In manufacture of benzoic acid, cinnamic acid, cinnamaldehyde, Schiff's base, etc.
(5) Tests : (i) Benzaldehyde forms a white precipitate with \[NaHS{{O}_{3}}\] solution.
(ii) Benzaldehyde forms a yellow precipitate with 2 : 4 dinitrophenyl hydrazine.
(iii) Benzaldehyde gives pink colour with Schiff's reagent.
(iv) Benzaldehyde forms black precipitate or silver mirror with Tollen's reagent.
(v) Benzaldehyde on treatment with alkaline \[KMn{{O}_{4}}\] and subsequent acidification gives a white precipitate of benzoic acid on cooling.
You need to login to perform this action.
You will be redirected in
3 sec
