Translation Or Protein Synthesis
Category : 12th Class
Formation of protein from mRNA is called translation is also known as polypeptide synthesis or protein synthesis. It is unidirectional process. The ribosomes of a polyribosome are held together by a strand of mRNA. Each eukaryotic ribosome has two parts, smaller 40S subunit (30S in prokaryotes) and larger 60S subunit (50S in prokaryotes).
Larger subunit has a groove for protection and passage of polypeptide, site A (acceptor or aminoacyl site), enzyme peptidyl transferase and a binding site for tRNA. The smaller subunit has a point for attachment of mRNA. Along with larger subunit, it forms a P-site or peptidyl transfer (donor site).
There are binding sites for initiation factors, elongation factors, translocase, GTPase, etc. The raw materials for protein synthesis are amino acids.mRNA, tRNAs and amino acyl tRNA synthetases.
Amino acids : Twenty types of amino acids and amides constitute the building blocks of proteins.
mRNA : It carries the coded information for synthesis of one (monocistronic) or more polypeptides (polycistronic). Its codons are recognised by tRNAs.
tRNAs : They picks up specific amino acid from amino acid pool and carrying over the mRNA strand.
Amino Acyl tRNA Synthetases : The enzymes are specific for particular amino acids and their tRNAs.
Activation of Amino Acids : An amino acid combines with its specific aminoacyl tRNA synthetase enzyme (AA-activating enzyme) in the presence of ATP to form aminoacyl adenylate enzyme complex (AA-AMP-E).
Pyrophosphate is released. Amino acid present in the complex is activated amino acid. It can attach to CCA or 3¢ end of its specific tRNA to form aminoacyl or AA-tRNA (charged tRNA / adaptor molecule).
Amino Acid (AA) + ATP + Aminoacyl tRNA Synthetase (E) \[\underset{\begin{smallmatrix} \text{amino acid adenylate} \\\text{enzyme complex}\end{smallmatrix}}{\mathop{\to \,\text{AA}-\text{AMP}-\text{E}}}\,\,+\text{PPi}\]\[AA-AMP-E+tRNA\to AAtRNA+AMP+Enzyme.\]
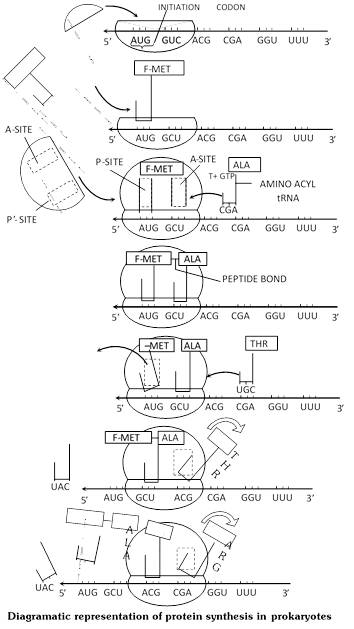
Initiation : It is accomplished with the help of initiation factors. Prokaryotes have three initiation factors \[\text{ }I{{F}_{3}},\text{ }I{{F}_{2}}\] and \[I{{F}_{1}}.\]Eukaryotes have nine initiation factors \[\text{ }eI{{F}_{1}},\text{ }eI{{F}_{2}},\text{ }eI{{F}_{3}},\text{ }eI{{F}_{4A}},\text{ }eI{{F}_{4B}},\text{ }eI{{F}_{4C}},\text{ }eI{{F}_{4D}},\text{ }eI{{F}_{5}},\text{ }eI{{F}_{6,}},\,\,mRNA\]attaches itself to smaller subunit of ribosome with its cap coming in contact with 3¢ end of 18 S rRNA (16S RNA in prokaryotes).
It requires \[eI{{F}_{2}}\](\[I{{F}_{3}}\] in prokaryotes). The initiation codon AUG or GUG comes to lie over P-site. It produces 40S – mRNA complex. P-site now attracts met tRNA (depending upon initiation codon). The anticodon of tRNA (UAC or CAC) comes to lie opposite initiation codon. Initiation factor \[eI{{F}_{3}}\](\[I{{F}_{2}}\] in prokaryotes) and GTP are required. It gives rise to 40S-mRNA \[-\text{ }tRN{{A}^{Met}}.\] Methionine is nonformylated \[(tRNA\,_{m}^{Met})\] in eukaryotic cytoplasm and formylated \[(tRNA\,_{f}^{Met})\] in case of prokaryotes.
The larger subunit of ribosome now attaches to 40S-mRNA-tRNAMet complex to form 80S mRNA -tRNA complex. Initiation factors \[eI{{F}_{1}}\] and \[eI{{F}_{4}}\] (A, B and C) are required in eukaryotes and \[I{{F}_{1}}\] in prokaryotes. \[M{{g}^{2+}}\] is essential for union of the two subunit of ribosomes. A-site becomes operational. Second codon of mRNA lies over it.
Elongation/chain formation : A new AA-tRNA comes to lie over the A site codon by means of GTP and elongation factor (\[eE{{F}_{1}}\] in eukaryotes, \[EF-Tu\] and \[EF-Ts\] in prokaryotes). Peptide bond \[(CO.NH)\] is established between carboxyl group \[(COOH)\] of amino acid of P-site and amino group \[(N{{H}_{2}})\] of amino acid at A-site with the help of enzyme peptidyl transferase/synthetase.
Connection between tRNA and amino acid of P-site and A-site tRNA comes to bear a dipeptydl. Free tRNA of P-site slips away. By means of translocase (\[eE{{F}_{2}}\]in eukaryotes and EF-G in prokaryotes) and GTP, ribosome moves in relation to mRNA so that peptidyl carrying tRNA comes to lie on P-site and a new codon is exposed at A-site. Incorporation of an amino acid in polypeptide chain thus requires one ATP and two GTP molecules. Peptide formation and translocation continue uninterrupted till the whole m-RNA code is translated into polypeptide. In a polyribosome, when a number of ribosomes are helping in translation of same mRNA code, the ribosome nearest the 5¢ end of mRNA carries the smallest polypeptide and the one towards the 3¢ end the longest. Of course, ultimately the whole polypeptide is formed by each.
Termination : Polypeptide synthesis stops when a nonsense or termination codon [UAA, (ochre), UAG (Amber) or UGA (opal)] reaches A-site. It does not attract any AA-tRNA, P-site tRNA seperates from its amino acid in the presence of release factor eRF1 in eukaryotes (RF1for UAG and UAA, RF2 for UAA and UGA in prokaryotes). The completed polypeptide is released, mRNA and ribosome separate. The two subunits of ribosome also dissociate with the help of dissociation factor.
Modification : Formylated methionine present at the beginning of polypeptide in prokaryotes and organelles is either deformylated (enzyme deformylase) or removed from chain (enzyme exopeptidase). Initially the polypeptide is elongated having only primary structure. As soon as the polypeptide comes out the groove of larger ribosome sub-unit, it forms \[\alpha -\]helix (secondary structure) which coils further forming a number of linkages (tertiary structure). Two or more polypeptides may get associated to become \[\beta -\]pleated which then coil to produce tertiary and quaternary structure.
You need to login to perform this action.
You will be redirected in
3 sec
