Some Important Terms
Category : JEE Main & Advanced
(1) Evaporation : Vaporisation occurring from the free surface of a liquid is called evaporation. Evaporation is the escape of molecules from the surface of a liquid. This process takes place at all temperatures and increases with the increase of temperature. Evaporation leads to cooling because the faster molecules escape and, therefore, the average kinetic energy of the molecules of the liquid (and hence the temperature) decreases.

(2) Melting (or fusion)/freezing (or solidification) : The phase change of solid to liquid is called melting or fusion. The reverse phenomenon is called freezing or solidification.

When pressure is applied on ice, it melts. As soon as the pressure is removed, it freezes again. This phenomenon is called regelation.
(3) Vaporisation/liquefication (condensation) : The phase change from liquid to vapour is called vaporisation. The reverse transition is called liquefication or condensation.
(4) Sublimation : Sublimation is the conversion of a solid directly into vapours. Sublimation takes place when boiling point is less than the melting point. A block of ice sublimates into vapours on the surface of moon because of very very low pressure on its surface. Heat required to change unit mass of solid directly into vapours at a given temperature is called heat of sublimation at that temperature.
(5) Hoar frost : Direct conversion of vapours into solid is called hoar frost. This process is just reverse of the process of sublimation, e.g., formation of snow by freezing of clouds.

(6) Vapour pressure : When the space above a liquid is closed, it soon becomes saturated with vapour and a dynamic equilibrium is established. The pressure exerted by this vapour is called Saturated Vapour Pressure (S.V.P.) whose value depends only on the temperature ? it is independent of any external pressure. If the volume of the space is reduced, some vapour liquefies, but the pressure is unchanged.
A saturated vapour does not obey the gas law whereas the unsaturated vapour obeys them fairly well. However, a vapour differs from a gas in that the former can be liquefied by pressure alone, whereas the latter cannot be liquefied unless it is first cooled.
(7) Boiling : As the temperature of a liquid is increased, the rate of evaporation also increases. A stage is reached when bubbles of vapour start forming in the body of the liquid which rise to the surface and escape. A liquid boils at a temperature at which the S.V.P. is equal to the external pressure.

It is a fast process. The boiling point changes on mixing impurities.
(8) Dew point : It is that temperature at which the mass of water vapour present in a given volume of air is just sufficient to saturate it, i.e. the temperature at which the actual vapour pressure becomes equal to the saturated vapuor pressure.
(9) Humidity : Atmospheric air always contains some water vapour. The mass of water vapour per unit volume is called absolute humidity.
The ratio of the mass of water vapour (m) actually present in a given volume of air to the mass of water vapour (M) required to saturate the same volume at the same temperature is called the relative humidity (R.H.). Generally, it is expressed as a percentage,
i.e., \[\text{R}\text{.H}\text{.}(%)=\frac{m}{M}\times 100(%)\]
R.H. May also be defined as the ratio of the actual vapour pressure (p) of water at the same temperature, i.e. \[\text{R}\text{.H}\text{.}(%)=\frac{p}{P}\times 100(%)\]
Thus R.H. may also be defined as
\[\text{R}\text{.H}\text{.}(%)=\frac{\text{S}\text{.V}\text{.P}\text{. at dew point}}{\text{S}\text{.V}\text{. P}\text{. at given temperature}}\times 100\]
(10) Variation of melting point with pressure : For those substances with contract on melting (e.g. water and rubber), the melting point decreases with pressure. The reason is the pressure helps shrinking and hence melting. Most substances expand on melting. (e.g. max, sulpher etc.)
An increase of pressure opposes the melting of such substances and their melting point is raised.
(11) Variation of latent heat with temperature and pressure : The latent heat of vapourization of a substance varies with temperature and hence pressure because the boiling point depends on pressure. It increases as the temperature is decreased. For example, water at 1 atm boils at \[{{100}^{o}}C\] and has latent heat \[2259\,\,J{{q}^{-1}}\] but at 0.5 atm it boils at \[{{82}^{o}}C\] and has latent heat \[2310\,\,J{{q}^{-1}}\]
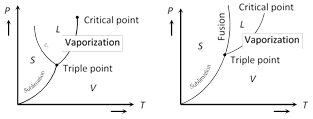
The latent heat of fusion shows similar but less pronounced pressure dependence.
The figures show the P-T graphs for (a) a substance (e.g., water) which contracts on melting an (b) a substance (e.g. wax) which expands on melting. The P-T graph consists of three curves.
(i) Sublimation curve which connects points at which vapour (V) and solid (S) exist in equilibrium.
(ii) Vapourization curve which shows vapour and liquid (L) existing in equilibrium.
(iii) Fusion curve which shows liquid and solid existing in equilibrium.
The three curves meet at a single point which is called the triple point. It is that unique temperature-pressure point for a substance at which all the three phases exist in equilibrium.
(12) Freezing mixture : If salt is added to ice, then the temperature of mixture drops down to less than \[{{0}^{o}}C\]. This is so because, some ice melts down to cool the salt to \[{{0}^{o}}C\]. As a result, salt gets dissolved in the water formed and saturated solution of salt is obtained; but the ice point (freeing point) of the solution formed is always less than that of pure water. So, ice cannot be in the solid state with the salt solution at \[{{0}^{o}}C\]. The ice which is in contact with the solution, starts melting and it absorbs the required latent heat from the mixture, so the temperature of mixture falls down.
You need to login to perform this action.
You will be redirected in
3 sec
