Alkali Metals And Their Compounds
Category : JEE Main & Advanced
The group 1 of the periodic table contains six elements, namely lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr). All these elements are typical metals. Francium is radioactive with longest lived isotope ![]() with half life period of only 21 minute. These are usually referred to as alkali metals since their hydroxides form strong bases or alkalies.
with half life period of only 21 minute. These are usually referred to as alkali metals since their hydroxides form strong bases or alkalies.
(1) Electronic configuration
| Elements | Discovery | Electronic configuration (\[n{{s}^{1}}\]) |
| \[_{3}\text{Li}\] | Arfwedson (1817) | \[{{[\text{He}]}^{2}}2{{s}^{1}}\] |
| \[_{11}\text{Na}\] | Davy (1807) | \[{{[\text{Ne }\!\!]\!\!\text{ }}^{\text{10}}}3{{s}^{1}}\] |
| \[_{19}\text{K}\] | Davy (1807) | \[{{[\text{Ar }\!\!]\!\!\text{ }}^{\text{18}}}4{{s}^{1}}\] |
| \[_{37}\text{Rb}\] | Bunsen (1861) | \[{{[\text{Kr }\!\!]\!\!\text{ }}^{\text{36}}}5{{s}^{1}}\] |
| \[_{55}\text{Cs}\] | Bunsen (1860) | \[{{[\text{Xe }\!\!]\!\!\text{ }}^{\text{54}}}6{{s}^{1}}\] |
| \[_{87}\text{Fr}\] | Percy (1939) | \[{{[\text{Rn }\!\!]\!\!\text{ }}^{\text{86}}}7{{s}^{1}}\] |
(2) Occurrence : Alkali metals are very reactive and thus found in combined state some important ores of alkali metals are given ahead.
(i) Lithium : Triphylite, Petalite, lepidolite, Spodumene [LiAl(SiO3)3], Amblygonite [Li(Al F)PO4]
(ii) Sodium : Chile salt petre (NaNO3), Sodium chloride (NaCl), Sodium sulphate (Na2SO4), Borax (Na2B4O710H2O), Glauber salt (Na2 SO4.10H2O)
(iii) Potassium : Sylime (KCl), carnallite (KCl.MgCl2.6H2O) and Felspar (K2O.Al2O3.6SiO2)
(iv) Rubidium : Lithium ores Lepidolite, triphylite contains 0.7 to 3% Rb2O
(v) Caesium : Lepidolite, Pollucite contains 0.2 to 7% Cs2O
(3) Extraction of alkali metals : Alkali metals cannot be extracted by the usual methods for the extraction of metals due to following reasons.
(i) Alkali metals are strong reducing agents, hence cannot be extracted by reduction of their oxides or other compounds.
(ii) Being highly electropositive in nature, it is not possible to apply the method of displacing them from their salt solutions by any other element.
(iii) The aqueous solutions of their salts cannot be used for extraction by electrolytic method because hydrogen ion is discharged at cathode instead of an alkali metal ions as the discharge potentials of alkali metals are high. However, by using Hg as cathode, alkali metal can be deposited. The alkali metal readily combines with Hg to form an amalgam from which its recovery difficult. The only successful method, therefore, is the electrolysis of their fused salts, usually chlorides. Generally, another metal chloride is added to lower their fusion temperature.
Fused NaCl : \[NaCl\xrightarrow{fusion}N{{a}^{+}}+C{{l}^{}}\]
\[\begin{matrix}Electrolysis:Anode:\,2C{{l}^{-}}\to C{{l}_{2}}+2{{e}^{-}} \\of\,fused\,salt:Cathode:2N{{a}^{+}}+2{{e}^{-}}\to 2Na\\\end{matrix}\]
(4) Alloys Formation
(i) The alkali metals form alloys among themselves as well as with other metals.
(ii) Alkali metals also get dissolved in mercury to form amalgam with evolution of heat and the amalgamation is highly exothermic .
Physical properties
(1) Physical state
(i) All are silvery white, soft and light solids. These can be cut with the help of knife. When freshly cut, they have bright lustre which quickly tarnishes due to surface oxidation.
(ii) These form diamagnetic colourless ions since these ions do not have unpaired electrons, (i.e. M+ has ns0configuration). That is why alkali metal salts are colourless and diamagnetic.
(2) Atomic and ionic radii
(i) The alkali metals have largest atomic and ionic radii than their successive elements of other groups belonging to same period.
(ii) The atomic and ionic radii of alkali metals, however, increases down the group due to progressive addition of new energy shells.
No doubt the nuclear charge also increases on moving down the group but the influence of addition of energy shell predominates
| Li | Na | K | Rb | Cs | Fr | |
| Atomic radius (pm) | 152 | 186 | 227 | 248 | 265 | 375 |
|
Ionic radius of M+ ions (pm) |
60 | 95 | 133 | 148 | 169 | - |
(3) Density
(i) All are light metals, Li, Na and K have density less than water. Low values of density are because these metals have high atomic volume due to larger atomic size. On moving down the group the atomic size as well as atomic mass both increase but increase in atomic mass predominates over increase in atomic size or atomic volume and therefore the ratio mass/volume i.e. density gradually increases down the groups
(ii) The density increases gradually from Li to Cs, Li is lightest known metal among all.
Li = 0.534, Na = 0.972, K = 0.86, Rb = 1.53 and Cs = 1.87 g/ml at 200C.
(iii) K is lighter than Na because of its unusually large atomic size.
(iv) In solid state, they have body centred cubic lattice.
(4) Melting point and Boiling point
(i) All these elements possess low melting point and boiling point in comparison to other group members.
| Li | Na | K | Rb | Cs | Fr | |
| melting point (K) | 453.5 | 370.8 | 336.2 | 312.0 | 301.5 | - |
|
boiling point (K) |
1620 | 1154.4 | 1038.5 | 961.0 | 978.0 | - |
(ii) The lattice energy of these atoms in metallic crystal lattice relatively low due to larger atomic size and thus possess low melting point and boiling point on moving down the group, the atomic size increases and binding energy of their atoms in crystal lattice decreases which results lowering of melting point.
(iii) Lattice energy decreases from Li to Cs and thus melting point and boiling also decreases from Li to Cs.
(5) Ionisation energy and electropositive or metallic character
(i) Due to unpaired lone electron in ns sub-shell as well as due to their larger size, the outermost electron is far from the nucleus, the removal of electron is easier and these low values of ionisation energy. (I.E.)
(ii) Ionisation energy of these metal decreases from Li to Cs.
| Ionisation energy | Li | Na | K | Rb | Cs | Fr |
| IE1 | 520 | 495 | 418 | 403 | 376 | – |
| IE2 | 7296 | 4563 | 3069 | 2650 | 2420 | – |
A jump in 2nd ionisation energy (huge difference) can be explained as,
\[Li\,:\,1{{s}^{2}}2{{s}^{1}}\underset{2s\,electron}{\overset{\operatorname{Re}movalof}\mathop{\xrightarrow{{}}}}}\,L{{i}^{+}}\,:\,1{{s}^{2}}\underset{1s\,electron\,\,}{\overset{\operatorname{Re}moval\,of}{\mathop{\xrightarrow{{}}}}}\,L{{i}^{2+}}:1{{s}^{1}}\]
Removal of 1s electrons from Li+ and that too from completely filled configuration requires much more energy and a jump in 2nd ionisation is noticed.
(iii) Lower are ionisation energy values, greater is the tendency to lose ns1 electron to change in M+ ion (i.e. M \[\to \] M++e–) and therefore stronger is electropositive character.
(iv) Electropositive character increases from Li to Cs.
Due to their strong electropositive character, they emit electrons even when exposed to light showing photoelectric effect. This property is responsible for the use of Cs and K in photoelectric cell.
(6) Oxidation number and valency
(i) Alkali metals are univalent in nature due to low ionisation energy values and form ionic compounds. Lithium salts are, however, covalent.
(ii) Further, the M+ ion acquires the stable noble gas configuration. It requires very high values of energy to pull out another electron from next to outer shell of M+ ion and that is why their second ionisation energy is very high. Consequently, under ordinary conditions, it is not possible for these metals to form M2+ ion and thus they show +1 oxidation state.
(iii) Since the electronic configuration of M+ ions do not have unpaired electron and thus alkali metal salts are diamagnetic and colourless. Only those alkali metal salts are coloured which have coloured anions e.g. K2Cr2O7 is orange because of orange coloured Cr2O72- ion, KMnO4 is violet because of violet coloured MnO41- ion.
(7) Hydration of Ions
(i) Hydration represents for the dissolution of a substance in water to get adsorb water molecule by weak valency force. Hydration of ions is the exothermic process (i.e energy is released during hydration) when ions on dissolution water get hydration.
(ii) The energy released when 1 mole of an ion in the gaseous state is dissolved in water to get it hydrated is called hydration energy \[{{M}_{(g)}}{{\,}^{+}}\,+Aq\to {{M}^{+}}_{_{(aq)\,}};\Delta H=\,ve.\]
(iii) Smaller the cation, greater is the degree of hydration. Hydration energy is in the order of, Li+ > Na+ > K+ > Rb+ > Cs+
(iv) Li+ being smallest in size has maximum degree of hydration and that is why lithium salts are mostly hydrated, LiCl. 2H2O also lithium ion being heavily hydrated, moves very slowly under the influence of electric field and, therefore, is the poorest conductor current among alkali metals ions It may, therefore, be concluded that it is the degree of hydration as well as the size of ion is responsible for the current carried by an ion.
| Relative ionic radii | Cs+ > Rb+ > K+ > Na+ > Li+ |
| Relative hydrated ionic radii | Li+ > Na+ > K+ > Rb+ > Cs+ |
| Relative conducting power | Cs+ > Rb+ > K+ > Na + > Li+ |
(8) Electronegativity, Electro positivity and metallic character.
(i) These metals are highly electropositive and thereby possess low values of electronegativities. Metallic character and electro positivity increase from Li to Cs (Li < Na < K < Rb < Cs)
(ii) Electronegativity of alkali metals decreases down the group as the trend of numerical values of electronegativity given below on Pauling scale suggests.
| Li | Na | K | Rb | Cs | Fr | |
| Electronegative | 0.98 | 0.93 | 0.82 | 0.82 | 0.79 | – |
Fr being radioactive elements and thus studies on physical properties of this element are limited.
(9) Specific heat : It decreases from Li to Cs.
| Li | Na | K | Rb | Cs | Fr | |
| Specific heat (Cal/g) | 0.941 | 0.293 | 0.17 | 0.08 | 0.049 | – |
(10) Conduction power : All are good conductors of heat and electricity, because of loosely held valence electrons.
(11) Standard oxidation potential and reduction properties
(i) Since alkali metals easily lose ns1 electron and thus they have high values of oxidation potential i.e.,
\[M+aq\to {{M}^{+}}_{_{(aq)}}+e\]
(ii) The standard oxidation potentials of a alkali metals (in volts) are listed below,
| Li | Na | K | Rb | Cs |
| + 3.05 | + 2.71 | + 2.93 | + 2.99 | + 2.99 |
(iii) More is oxidation potential, more is the tendency to get oxidized and thus more powerful is reducing nature in aqueous medium that is why alkali metals liberate H2 from H2O and HCl.
\[2{{H}_{2}}O+2M\to 2MOH+{{H}_{2}}\] ; \[2HCl+2M\to 2MCl+{{H}_{2}}\]
(iv) However, an examination of ionisation energy for alkali metals reveals that Li should have the minimum tendency to lose electron and thus its reducing nature should be minimum. The greatest reducing nature of Li in aq. medium is accounted due to the maximum hydration energy of Li+ ion. For Lithium
\[\frac{\begin{matrix}L{{i}_{(s)}}\to L{{i}_{(g)}}; & \Delta {{H}_{1}}=\text{ Heat of sublimation, }\Delta {{H}_{s}} \\L{{i}_{(g)}}\to L{{i}^{+}}_{_{(g)}}+e; & \Delta {{H}_{2}}=I{{E}_{1}} \\L{{i}^{+}}_{_{(g)}}\to L{{i}^{+}}_{_{(aq);}} & \Delta {{H}_{3}}=-\text{ Heat of hydration, }\Delta {{H}_{h}} \\\end{matrix}}{L{{i}_{(s)}}+ {{H}_{2}}O\to L{{i}^{+}}_{(aq)}+e;\Delta H=\Delta {{H}_{1}}+\Delta {{H}_{2}}+\Delta {{H}_{3}}=\Delta {{H}_{s}}+I{{E}_{1}}-\Delta {{H}_{h}}}\]
Similarly, for sodium,
\[N{{a}_{(s)}}+{{H}_{2}}O\to N{{a}^{+}}_{_{(aq)}}+e;\Delta H=\Delta {{H}_{(s)}}+I{{E}_{1}}-\Delta {{H}_{h}}\]
\[\Delta {{H}_{h}}\] for \[Li>\Delta {{H}_{h}}\] for Na. Therefore, large negative \[\Delta H\] values are observed in case of Li and this explains for more possibility of Li to get itself oxidized or have reducing nature.
(12) Characteristic flame colours : The alkali metals and their salts give characteristic colour to Bunsen flame. The flame energy causes and excitation of the outermost electron which on reverting back to its initial position gives out the absorbed energy as visible light. These colour differ from each other Li –crimson, Na–Golden yellow, K – Pale violet , Rb-Red violet and Cs –Blue violet. These different colours are due to different ionisation energy of alkali metals. The energy released is minimum in the case of Li+ and increases in the order.
| Energy released | : Li+ < Na+ < K+ < Rb+ < Cs+ |
| \[\lambda \] released | : Li+ > Na+ > K+ > Rb+ > Cs+ |
| Frequency released | : Li+ < Na+ < K+ < Rb+ < Cs+ |
Chemical properties
(1) Formation of oxides and hydroxides
(i) These are most reactive metals and have strong affinity for O2 quickly tranish in air due to the formation of a film of their oxides on the surface. These are, therefore, kept under kerosene or paraffin oil to protect them from air,
\[M+{{O}_{2}}\to \underset{\text{Oxide}}{\mathop{{{M}_{2}}O}}\,\xrightarrow{{}}\underset{\text{Peroxide}}{\mathop{{{M}_{2}}{{O}_{2}}}}\,\]
(ii) When burnt air (O2), lithium forms lithium oxide (Li2O) sodium forms sodium peroxide (Na2O2) and other alkali metals form super oxide (Mo2 i.e. KO2,RbO2 or CsO2)
\[2Li+\frac{1}{2}{{O}_{2}}\to \underset{\text{Lithuim}\ \text{oxide}}{\mathop{L{{i}_{2}}O}}\,\]; \[2Na+{{O}_{2}}\to N{{a}_{2}}\,{{O}_{2}}\]
\[\underset{{}}{\mathop{K+{{O}_{2}}}}\,\to \underset{\text{Potassium super oxide}}{\mathop{K{{O}_{2}}}}\,\]
The reactivity of alkali metals towards oxygen to form different oxides is due to strong positive field around each alkali metal cation. Li+ being smallest, possesses strong positive field and thus combines with small anion O2– to form stable Li2O compound. The Na+ and K+ being relatively larger thus exert less strong positive field around them and thus reacts with larger oxygen anion i.e, to form stable oxides.
The monoxide, peroxides and superoxides have O2 and ions respectively. The structures of each are,
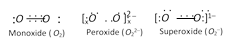
The O2–1 ion has a three electron covalent bond and has one electron unpaired. It is therefore superoxides are paramagnetic and coloured KO2 is light yellow and paramagnetic substance.
(iii) The oxides of alkali metals and metal itself give strongly alkaline solution in water with evolution of heat
\[M+{{H}_{2}}O\to MOH+\frac{1}{2}{{H}_{2}};\,\,\,\,\,\,\,\,\,\,\,\Delta H=-ve\]
\[L{{i}_{2}}O+{{H}_{2}}O\to 2LiOH;\,\,\,\,\,\,\,\,\,\,\,\,\Delta H=-ve\]
\[N{{a}_{2}}{{O}_{2}}+2{{H}_{2}}O\to 2NaOH+{{H}_{2}}{{O}_{2(l)}}\ ;\,\,\,\,\,\,\,\,\,\Delta H=-ve\]
\[2K{{O}_{2}}+2{{H}_{2}}O\to 2KOH+{{H}_{2}}{{O}_{2(l)}}+{{O}_{2(g)\,}};\,\,\,\,\,\,\,\,\,\,\,\Delta H=-ve\]
The peroxides and superoxides act as strong oxidising agents due to formation of H2O2
(iv) The reactivity of alkali metals towards air and water increases from Li to Cs that is why lithium decomposes H2O very slowly at 25oC whereas Na does so vigorously, K reacts producing a flame and Rb, Cs do so explosively.
\[M+{{H}_{2}}O\to MOH+\frac{1}{2}{{H}_{2}}\]
(v) The basic character of oxides and hydroxides of alkali metals increases from Li to Cs. This is due to the increase in ionic character of alkali metal hydroxides down the group which leads to complete dissociation and leads to increase in concentration of OH– ions.
(2) Hydrides
(i) These metals combine with H to give white crystalline ionic hydrides of the general of the formula MH.
(ii) The tendency to form their hydrides, basic character and stability decreases from Li to Cs since the electropositive character decreases from Cs to Li.
2M + H2 \[\to \] 2MH ; Reactivity towards H2 is Cs < Rb < K < Na < Li.
(iii) The metal hydrides react with water to give MOH and H2 ; MH + H2 O \[\to \] MOH + H2
(iv) The ionic nature of hydrides increases from Li to Cs because of the fact that hydrogen is present in the these hydrides as H– and the smaller cation will produce more polarisation of anion (according to Fajans rule) and will develop more covalent character.
(v) The electrolysis of fused hydrides give H2 at anode.
At cathode: Na+ +e– \[\to \] Na; At anode: \[{{H}^{-}}\to \frac{1}{2}H_{2}^{{}}+{{e}^{-}}\]
(vi) Alkali metals also form hydrides like NaBH4, LiAlH4 which are good reducing agent.
(3) Carbonates and Bicarbonates
(i) The carbonates (M2CO3) & bicarbonates (MHCO3) are highly stable to heat, where M stands for alkali metals.
(ii) The stability of these salts increases with the increasing electropositive character from Li to Cs. It is therefore Li2CO3 decompose on heating, Li2CO3 \[\to \] Li2O+CO2
(iii) Bicarbonates are decomposed at relatively low temperature, \[2MHC{{O}_{3}}\xrightarrow{{{300}^{0}}C}{{M}_{2}}C{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\]
(iv) Both carbonates and bicarbonates are soluble in water to give alkaline solution due to hydrolysis of carbonate ions or bicarbonate ions.
(4) Halides
(i) Alkali metals combine directly with halogens to form ionic halide \[{{M}^{+}}{{X}^{-}}\].
(ii) The ease with which the alkali metals form halides increases from Li to Cs due to increasing electropositive character from Li to Cs.
(iii) Lithium halides however have more covalent nature. Smaller is the cation, more is deformation of anion and thus more is covalent nature in compound. Also among lithium halides, lithium iodide has maximum covalent nature because of larger anion which is easily deformed by a cation. Thus covalent character in lithium halides is, LiI > LiBr > LiCl > LiF
(iv) These are readily soluble in water. However, lithium fluoride is sparingly soluble. The low solubility of LiF is due to higher forces of attractions among smaller Li+ and smaller F– ions (high lattice energy).
(v) Halides having ionic nature have high m.pt. and good conductor of current. The melting points of halides shows the order, NaF > NaCl > NaBr > Nal
(vi) Halides of potassium, rubidium and caesium have a property of combining with extra halogen atoms forming polyhalides.
KI + I2 \[\to \] KI3 ; In KI3(aq) the ions K+ and I–3 are present
(5) Solubility in liquid NH3
(i) These metals dissolve in liquid NH3 to produce blue coloured solution, which conducts electricity to an appreciable degree.
(ii) With increasing concentration of ammonia, blue colour starts changing to that of metallic copper after which dissolution of alkali metals in NH3 ceases.
(iii) The metal atom is converted into ammoniated metal in i.e. M+ (NH3) and the electron set free combines with NH3 molecule to produce ammonia solvated electron.
\[Na+(x+y)N{{H}_{3}}\to \underset{\text{Ammoniated cation}}{\mathop{{{[Na{{(N{{H}_{3}})}_{x}}]}^{+}}}}\,+\underset{\text{Ammoniated}\ \text{electron}}{\mathop{{{[e{{(N{{H}_{3}})}_{y}}]}^{-}}}}\,\]
(iv) It is the ammoniated electron which is responsible for blue colour, paramagnetic nature and reducing power of alkali metals in ammonia solution. However, the increased conductance nature of these metals in ammonia is due to presence of ammoniated cation and ammonia solvated electron.
(v) The stability of metal-ammonia solution decreases from Li to Cs.
(vi) The blue solution on standing or on heating slowly liberates hydrogen, 2M + 2NH3 \[\to \] 2MNH2 + H2. Sodamide (NaNH2) is a waxy solid, used in preparation of number of sodium compounds.
(6) Nitrates : Nitrates of alkali metals (MNO3) are soluble in water and decompose on heating. LiNO3 decomposes to give NO2 and O2 and rest all give nitrites and oxygen.
2MNO3 \[\to \] 2MNO2 + O2 (except Li)
4 LiNO3 \[\to \] 2Li2O + 4NO2 + O2
(7) Sulphates
(i) Alkali metals’ sulphate have the formula M2SO4 .
(ii) Except Li2SO4, rest all are soluble in water.
(iii) These sulphates on fusing with carbon form sulphides, M2SO4 + 4C \[\to \] M2S + 4CO
(iv) The sulphates of alkali metals (except Li) form double salts with the sulphate of the trivalent metals like Fe, Al, Cr etc. The double sulphates crystallize with large number of water molecules as alum. e.g. K2SO4 . Al2 (SO4)3. 24 H2O.
(8) Reaction with non-metals
(i) These have high affinity for non-metals. Except carbon and nitrogen, they directly react with hydrogen, halogens, sulphur, phosphorus etc. to form corresponding compounds on heating.
\[2Na+{{H}_{2}}\xrightarrow{{{300}^{0}}C}2NaH;\,\,2K+{{H}_{2}}\to 2KH\]
\[2Na+C{{l}_{2}}\to 2NaCl\,;\,2K+C{{l}_{2}}\to 2KCl\]
\[2Na+S\to N{{a}_{2}}S\] \[;\,\,2K+S\to {{K}_{2}}S\]
\[3Na+P\to N{{a}_{3}}P\] \[;\,3K+P\to {{K}_{3}}P\]
(ii) Li reacts, however directly with carbon and nitrogen to form carbides and nitrides.
2Li + 2C \[\to \] LiC2 ; 6Li + 2N2 \[\to \] 2 Li3N
(iii) The nitrides of these metals on reaction with water give NH3.
M3N + 3H2O \[\to \] 3MOH + NH3
(9) Reaction with acidic hydrogen : Alkali metals react with acids and other compounds containing acidic hydrogen (i.e, H atom attached on F,O, N and triply bonded carbon atom, for example, HF, H2O, ROH, RNH2, CH \[\equiv \] CH) to liberate H2 .
\[M+{{H}_{2}}O\to MOH+\frac{1}{2}{{H}_{2}}\] ; \[M+HX\to MX+\frac{1}{2}{{H}_{2}}\]
\[M+ROH\to ROH+\frac{1}{2}{{H}_{2}}\]; \[M+RN{{H}_{2}}\to RNHNa+\frac{1}{2}{{H}_{2}}\]
(10) Complex ion formation : A metal shows complex formation only when it possesses the following characteristics, (i) Small size (ii) High nuclear charge (iii) Presence of empty orbitals in order to accept electron pair ligand. Only Lithium in alkali metals due to small size forms a few complex ions Rest all alkali metals do not possess the tendency to form complex ion.
Anomalous behaviour of Lithium
Anomalous behaviour of lithium is due to extremely small size of lithium its cation on account of small size and high nuclear charge, lithium exerts the greatest polarizing effect out of all alkali metals on negative ion. Consequently lithium ion possesses remarkable tendency towards solvation and develops covalent character in its compounds. Li differs from other alkali metals in the following respects,
(1) It is comparatively harder than other alkali metals. Li can’nt be stored in kerosene as it floats to the surface, due to its very low density. Li is generally kept wrapped in parrafin wax.
(2) It can be melted in dry air without losing its brilliance.
(3) Unlike other alkali metals, lithium is least reactive among all. It can be noticed by the following properties,
(i) It is not affected by air. (ii) It decomposes water very slowly to liberate H2. (iii) It hardly reacts with bromine while other alkali metals react violently.
(4) Lithium is the only alkali metal which directly reacts with N2 to form Lithium nitride (Li3N)
(5) Lithium when heated in NH3 forms amide, Li2 NH while other metals form amides, MNH2.
(6) When burnt in air, lithium form Li2O sodium form Na2O and Na2O2 other alkali metals form monoxide, peroxide and superoxide.
(7) Li2O is less basic and less soluble in water than other alkali metals.
(8) LiOH is weaker base than NaOH or KOH and decomposes on heating.
\[2LiOH\xrightarrow{\Delta }L{{i}_{2}}O+{{H}_{2}}O\]
(9) LiHCO3 is liquid while other metal bicarbonates are solid.
(10) Only Li2CO3 decomposes on heating
\[L{{i}_{2}}C{{O}_{3}}\xrightarrow{heat}L{{i}_{2}}O+C{{O}_{2}}\]
Na2CO3, K2CO3 etc. do not decompose on heating.
(11) LiNO3 and other alkali metal nitrates give different products on heating
4LiNO3 = 2Li2O+4NO2 + O2 ; 2NaNO3 = 2NaNO2 + O2
(12) LiCl and LiNO3 are soluble in alcohol and other organic solvents. These salts of other alkali metals are, however, insoluble in organic solvents.
(13) LiCl is deliquescent while NaCl, KBr etc. are not. Lithium chloride crystals contain two molecules of water of crystallisation (LiCl. 2H2O). Crystals of NaCl KBr, KI etc do not conation water of crystallisation.
(14) Li2SO4 does not form alums like other alkali metals.
(15) Li reacts with water slowly at room temperature Na reacts vigorously Reaction with K. Rb and Cs is violent.
(16) Li reacts with Br2 slowly. Reaction of other alkali metals with Br2 is fast.
(17) Li2 CO3 Li2C2O4, LiF , Li3PO4 are the only alkali metal salts which are insoluble or sparingly soluble in water.
Diagonal Relationship of Li with Mg
Due to its small size lithium differs from other alkali metals but resembles with Mg as its size is closer to Mg Its resemblance with Mg is known as diagonal relationship. Generally the periodic properties show either increasing or decreasing trend along the group and vice versa along the period which brought the diagonally situated elements to closer values. Following are the characteristic to be noted.
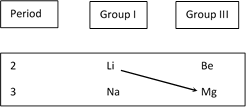
(1) Both Li and Mg are harder and higher m.pt than the other metals of their groups.
(2) Due to covalent nature, chlorides of both Li and Mg are deliquescent and soluble in alcohol and pyridine while chlorides of other alkali metals are not so.
(3) Fluorides, phosphates of Li and Mg are sparingly soluble in water whereas those of other alkali metals are soluble in water.
(4) Carbonates of Li and Mg decompose on heating and liberate CO2 Carbonates of other alkali metals are stable towards heat and decomposed only on fusion.
Li2CO3 \[\to \] Li2O + CO2 ; Mg CO3 \[\to \] MgO + CO2
(5) Hydroxides and nitrates of both Li and Mg decompose on heating to give oxide. Hydroxides of both Li and Mg are weak alkali.
4 LiNO3 \[\to \] 2Li2O + 4NO2 + O2
2Mg(NO3)2 \[\to \] 2MgO + 4NO2 + O2
2LiOH \[\to \] Li2O + H2O ; Mg(OH)2 \[\to \] MgO + H2O
Hydroxides of other alkali metals are stable towards heat while their nitrates give O2 and nitrite.
2KNO3 \[\to \] 2KNO2 + O2
(6) Both Li and Mg combine directly with N2 to give nitrides Li3N and Mg3N2. Other alkali metals combine at high temperature, 6Li + N2 \[\to \] 2Li3N; 3Mg + N2 \[\to \] Mg3N2. Both the nitrides are decomposed by water to give NH3
Li3N + 3H2O \[\to \] 3LiOH + NH3 ;
Mg3N2 + 6H2O \[\to \] 3Mg(OH)2+ 2NH3
(7) Bicarbonates of Li and Mg are more soluble in water than carbonates whereas carbonates of alkali metals are more soluble.
(8) Both Li and Mg combine with carbon on heating.
2Li + 2C \[\to \] Li2C2 ; Mg + 2C ® Mg C2
(9) The periodic properties of Li and Mg are quite comparable
| Li | Mg | |
| Electronegativity | 1.0 | 1.2 |
| Atomic radii | 1.23 | 1.36 |
| Ionic radii | 0.60 (Li+) | 0.65 (Mg+2) |
| Atomic volume | 12.97 c.c | 13.97 c.c |
(10) Both have high polarizing power.
Polarizing Power = Ionic charge / (ionic radius)2.
(11) Li and Mg Form only monooxide on heating in oxygen.
4Li + O2 \[\to \] 2 Li2O ; 2Mg + O2 \[\to \] 2 MgO
(12) Li2SO4 like MgSO4 does not form alums.
(13) The bicarbonates of Li and Mg do not exist in solid state, they exist in solution only.
(14) Alkyls of Li and Mg (R. Li and R.MgX) are soluble in organic solvent.
(15) Lithium chloride and MgCl2 both are deliquescent and separate out from their aqueous solutions as hydrated crystals, LiCl. 2H2O and MgCl2 . 2H2O.
Uses of Lithium
(1) It is used as a deoxidiser in metallurgy of Cu and Ni.
(2) It is used as an alloying metal with
(i) Pb to give toughened bearings.
(ii) Al to give high strength Al-alloy for aircraft industry.
(iii) Mg (14% Li) to give extremely tough and corrosion resistant alloy which is used for armour plate in aerospace components.
Sodium and its compounds
(1) Ores of sodium : (common salt), (chile salt petre), (Glauber's salt), borax (sodium tetraborate or sodium borate, .
(2) Extraction of sodium : It is manufactured by the electrolysis of fused sodium chloride in the presence of \[CaC{{l}_{2}}\] and using graphite anode and iron cathode. This process is called Down process.
\[NaCl\rightleftharpoons N{{a}^{+}}+C{{l}^{-}}\].
At cathode : \[N{{a}^{+}}+{{e}^{-}}\to Na\];
At anode : \[C{{l}^{-}}\to Cl+{{e}^{-}}\]; \[Cl+Cl\to C{{l}_{2}}\uparrow \]
Sodium cannot be extracted from aqueous because \[E_{{{H}_{2}}O/{{H}_{2}}}^{0}\] (–0.83V) is more than \[{{E}^{0}}N{{a}^{+}}/Na\] (–2.71V).
Anode and cathode are separated by means of a wire gauze to prevent the reaction between Na and \[C{{l}_{2}}\].
(3) Compound of sodium
(i) Sodium chloride : It is generally obtained by evaporation of sea water by sun light. However NaCl so obtained contains impurities like \[CaS{{O}_{4}},\,CaC{{l}_{2}}\] and \[MgC{{l}_{2}}\] which makes the salt deliquescent. It is then purified by allowing \[HCl\] gas to pass through the impure saturated solution of \[NaCl\]. The concentration of \[C{{l}^{-}}\] ions increases and as a result pure \[NaCl\] gets precipitated due to common ion effect.
(ii) Sodium hydroxide NaOH (Caustic soda)
Preparation
(a) Gossage process :
\[\underset{(10\text{ }\!\!%\!\!\text{ solution)}}{\mathop{N{{a}_{2}}C{{O}_{3}}}}\,+Ca{{(OH)}_{2}}\to 2NaOH\downarrow +CaC{{O}_{3}}\]
(b) Electrolytic method : Caustic soda is manufactured by the electrolysis of a concentrated solution of \[NaCl.\]
At anode: \[C{{l}^{-}}\]discharged; At cathode: \[N{{a}^{+}}\] discharged
(c) Castner - Kellener cell (Mercury cathode process) : \[NaOH\] obtained by electrolysis of aq. solution of brine. The cell comprises of rectangular iron tank divided into three compartments.
Outer compartment – Brine solution is electrolysed ; Central compartment – 2% \[NaOH\] solution and \[{{H}_{2}}\]
Properties : White crystalline solid, highly soluble in water, It is only sparingly soluble in alcohol.
(a) Reaction with salt :
\[FeC{{l}_{3}}+3NaOH\to \] \[\underset{\text{(Insoluble}\ \text{hydroxide)}}{\mathop{Fe{{(OH)}_{3}}}}\,\downarrow +\,\,\,\underset{{}}{\mathop{3NaCl}}\,\]
\[\underset{\,\,\,\,}{\mathop{HgC{{l}_{2}}+2NaOH\to 2NaCl\underset{\text{unstable}}{\mathop{\ +\ Hg{{(OH)}_{2}}}}\,\,\to \ \,{{\text{H}}_{\text{2}}}O+\underset{\text{yellow}}{\mathop{HgO\downarrow }}\,}}\,\]
\[AgN{{O}_{3}}+2NaOH\to 2NaN{{O}_{3}}+2AgOH\to \]\[\underset{\text{Brown}}{\mathop{A{{g}_{2}}O}}\,\downarrow +{{H}_{2}}O\]
\[Zn,\,Al,\,Sb,\,Pb,\,Sn\] and \[As\] forms insoluble hydroxide which dissolve in excess of \[NaOH\] (amphoteric hydroxide).
\[N{{H}_{4}}Cl+NaOH\xrightarrow{\text{heat}}NaCl+N{{H}_{3}}\uparrow +{{H}_{2}}O\]
(b) Reaction with halogens :
\[{{X}_{2}}+2NaOH\] (cold) \[\to \] \[NaX+\underset{\text{sod}\text{. hypohalite}}{\mathop{NaXO+{{H}_{2}}O}}\,\]
\[3{{X}_{2}}+6NaOH\](hot) \[\to \]\[5NaX+\underset{\text{(Sod}\text{. halate)}\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ }{\mathop{NaX{{O}_{3}}+3{{H}_{2}}O}}\,\]; \[(X=Cl,\,Br,\,I)\]
(c) Reaction with metals : Weakly electropositive metals like \[Zn,\,Al\] and \[Sn\] etc.
\[Zn+2NaOH\to N{{a}_{2}}Zn{{O}_{2}}+{{H}_{2}}\uparrow \]
(d) Reaction with sand, SiO2 :
\[2NaOH+Si{{O}_{2}}\to \] \[\underset{\text{Sod}\text{. silicate (glass)}}{\mathop{N{{a}_{2}}Si{{O}_{3}}}}\,+{{H}_{2}}O\]
(e) Reaction with CO:
\[NaOH+CO\underset{5-10\ atm}{\mathop{\xrightarrow{150-{{200}^{o}}C}}}\,\underset{\text{Sod}\text{. formate}}{\mathop{HCOONa}}\,\]
\[NaOH\] breaks down the proteins of the skin flesh to a pasty mass, therefore it is commonly known as caustic soda.
Caustic property : sodium hydroxide breaks down the proteins of the skin flesh to a pasty mass, therefore, it is commonly known as caustic soda.
Uses : Sodium hydroxide is used :
(a) in the manufacture of soidum metal, soap (from oils and fats), rayon, paper, dyes and drugs,
(b) for mercurinzing cotton to make cloth unshrinkable and
(c) as a reagent in the laboratory.
(iii) Sodium carbonate or washing soda, \[N{{a}_{2}}C{{O}_{3}}\]
It exists in various forms, namely anhydrous sodium carbonate Na2CO2 (soda-ash); monohydrate \[N{{a}_{2}}C{{O}_{3}}.{{H}_{2}}O\] (crystal carbonate); hyptahydrate \[N{{a}_{2}}C{{O}_{3}}.7{{H}_{2}}O\] and decahydrate \[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\] (washing soda or sal soda).
Preparation : (a) Solvay process : In this process, brine \[(NaCl)\], \[N{{H}_{3}}\] and \[C{{O}_{2}}\] are the raw materials.
\[N{{H}_{3}}+C{{O}_{2}}+{{H}_{2}}O\to \]\[N{{H}_{4}}HC{{O}_{3}}\]
\[N{{H}_{4}}HC{{O}_{3}}+NaCl\xrightarrow{{{30}^{o}}C}NaHC{{O}_{3}}\downarrow +N{{H}_{4}}Cl\]
\[2NaHC{{O}_{3}}\xrightarrow{{{250}^{o}}C}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\]
\[2N{{H}_{4}}Cl+\underset{\begin{smallmatrix} \text{slaked}\,\,\,\, \\ \,\text{lime}\end{smallmatrix}}{\mathop{Ca{{(OH)}_{2}}}}\,\to CaC{{l}_{2}}+2{{H}_{2}}O+2N{{H}_{3}}\]
\[CaC{{l}_{2}}\] so formed in the above reaction is a by product of solvay process.
Properties
(a) \[\underset{\text{(decahydrate)}}{\mathop{N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O}}\,\xrightarrow{\text{dry air}}\underset{\text{(Monohydrate)}}{\mathop{N{{a}_{2}}C{{O}_{3}}.{{H}_{2}}O}}\,+9{{H}_{2}}O\]
\[N{{a}_{2}}C{{O}_{3}}.\,{{H}_{2}}O\xrightarrow{\Delta }\,\,\,\,\,\,N{{a}_{2}}C{{O}_{3}}\text{ }\]
It does not decompose on funrther heating even to redness (m.pt. 853°C)
(b) It is soluble in water with considerable evolution of heat.
\[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\to \underset{\text{Weak}\,\text{acid }}{\mathop{{{H}_{2}}C{{O}_{3}}+}}\,2N{{a}^{+}}+2O{{H}^{-}}\]
(c) It is readily decomposed by acids with the evolution of \[C{{O}_{2}}\] gas.
(d) \[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\to 2NaHC{{O}_{3}}\]
Uses : In textile and petroleum refining, Manufacturing of glass, \[NaOH\] soap powders etc.
(iv) Sodium peroxide (Na2O2)
Preparation : It is manufactured by heating sodium metal on aluminium trays in air (free from\[C{{O}_{2}})\]
\[2Na+{{O}_{2}}\](air)\[\xrightarrow{\Delta }N{{a}_{2}}{{O}_{2}}\]
Properties : (a) When pure it is colourless. The faint yellow colour of commercial product is due to presence of small amount of superoxide \[(Na{{O}_{2}}).\]
(b) On coming with moist air it become white due to formation of \[NaOH\] and \[N{{a}_{2}}C{{O}_{3}}\].
\[2N{{a}_{2}}{{O}_{2}}+2{{H}_{2}}O\to 4NaOH+{{O}_{2}}\] ;
\[2NaOH+C{{O}_{2}}\to N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\]
(c) It is powerful oxidising agent. It oxidises \[Cr\](III) hydroxide to sodium chromate, \[Mn\](II) to sodium manganate and sulphides to sulphates.
Uses : As a bleaching agent and it is used for the purification of air in confined spaces such as submarines because it can combine with \[C{{O}_{2}}\] to give \[N{{a}_{2}}C{{O}_{3}}\] and oxygen, \[2C{{O}_{2}}+2N{{a}_{2}}{{O}_{2}}\]\[\to 2N{{a}_{2}}C{{O}_{3}}+{{O}_{2}}\].
(v) Micro cosmic salt [Na (NH4) HPO4. 4H2O]
Prepared by dissolving equimolar amounts of \[N{{a}_{2}}HP{{O}_{4}}\] and \[N{{H}_{4}}Cl\] in water in 1 : 1 ratio followed by crystallization
\[N{{H}_{4}}Cl+N{{a}_{2}}HP{{O}_{4}}\xrightarrow{{}}\underset{\downarrow \,Crystallization}{\mathop{Na(N{{H}_{4}})HP{{O}_{4}}+NaCl}}\,\]
\[\underset{\text{(Colourless crystal)}}{\mathop{Na(N{{H}_{4}})HP{{O}_{4}}.4{{H}_{2}}O}}\,\]
Chemical properties :
On heating M.C.S, \[NaP{{O}_{3}}\] is formed. \[NaP{{O}_{3}}\] forms coloured beads with oxides of transition metal cloudy \[Si{{O}_{2}}\]
\[Na(N{{H}_{4}})HP{{O}_{4}}\xrightarrow{\Delta }\underset{\begin{smallmatrix} \text{(Sodium meta} \\ \text{phosphate)}\end{smallmatrix}}{\mathop{NaP{{O}_{3}}}}\,+{{H}_{2}}O+N{{H}_{3}}\]
\[\underset{\begin{smallmatrix} \text{(Trans parent} \\ \text{glassy bead)}\end{smallmatrix}}{\mathop{NaP{{O}_{3}}}}\,+CuO\xrightarrow{\Delta }\,\underset{\text{(blue bead)}}{\mathop{CuNaP{{O}_{4}}}}\,\]
\[NaP{{O}_{3}}+CoO\xrightarrow{{}}CoNaP{{O}_{4}}\] (blue bend)
\[NaP{{O}_{3}}+MnO\xrightarrow{{}}NaMn{{O}_{4}}\] (blue bead)
Uses : (a) For the formation of sodium meta phosphate and copper sodium phosphate
(b) It is used for the detection of colured ion
(c) It is espacially used for testing silica with which a cloudy bead containing floating properties of silica is obtained.
(vi) Sodium bi Carbonate (NaHCO3, Baking soda)
Preparation : It is an inter mediate compound in manufacture of sodium carbonate by the solvay’s process
\[NaCl+N{{H}_{3}}+C{{O}_{2}}+{{H}_{2}}\xrightarrow{{}}NaHC{{O}_{3}}+N{{H}_{4}}Cl\]
Properties: \[2NaHC{{O}_{3}}\xrightarrow{50-{{100}^{o}}C}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+C{{O}_{2}}\]
It is amphiprotic \[HCO_{3}^{-}+{{H}^{+}}\]?\[{{H}_{2}}C{{O}_{3}}\]
\[HCO_{3}^{-}\] ?\[{{H}^{+}}+CO_{3}^{2-}\]
Uses : (a) Baking powder contains \[NaHC{{O}_{3}}\], \[Ca{{({{H}_{2}}P{{O}_{4}})}_{2}}\] and starch.
Improved Baking powder contains 40% starch 30% \[NaHC{{O}_{3}}\], 20% \[NaAl{{(S{{O}_{4}})}_{2}}\] and 10% \[Ca{{H}_{2}}(P{{O}_{4}})\]
(b) In pharmacentical industry (Antacids etc.)
(c) Fire extingerishers.
(vii) Sodium Sulphate Na2SO4 or salt cake
Preparation : It is the by-product of \[HCl\] industry
\[2NaCl+{{H}_{2}}S{{O}_{4}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{4}}+HCl\]
Properties : When aqueous solution of \[N{{a}_{2}}S{{O}_{4}}\] is cooled below \[{{32}^{o}}C\]Glauber’s salt \[(N{{a}_{2}}S{{O}_{4}}.10{{H}_{2}}O)\] gets crystallised and if cooled to \[{{12}^{o}}C\], \[N{{a}_{2}}S{{O}_{4}}7{{H}_{2}}O\] crystals are formed.
\[N{{a}_{2}}S{{O}_{4}}.10{{H}_{2}}O\xrightarrow{\text{(indry air)}}N{{a}_{2}}S{{O}_{4}}+10{{H}_{2}}O\]
Uses : \[N{{a}_{2}}S{{O}_{4}}\] finds use in paper industry detergent and glass manufacturing.
You need to login to perform this action.
You will be redirected in
3 sec
