Quantum Numbers
Category : JEE Main & Advanced
Each orbital in an atom is specified by a set of three quantum numbers (n, l, m) and each electron is designated by a set of four quantum numbers (n, l, m and s).
(1) Principle quantum number (n)
(i) It was proposed by Bohr and denoted by 'n'.
(ii) It determines the average distance between electron and nucleus, means it denotes the size of atom.
(iii) It determine the energy of the electron in an orbit where electron is present.
(iv) The maximum number of an electron in an orbit represented by this quantum number as \[2{{n}^{2}}.\] No energy shell in atoms of known elements possess more than 32 electrons.
(v) It gives the information of orbit K, L, M, N------------.
(vi) Angular momentum can also be calculated using principle quantum number
(2) Azimuthal quantum number (l)
(i) Azimuthal quantum number is also known as angular quantum number. Proposed by Sommerfield and denoted by 'l'.
(ii) It determines the number of sub shells or sublevels to which the electron belongs.
(iii) It tells about the shape of subshells.
(iv) It also expresses the energies of subshells \[s<p<d<f\] (increasing energy).
(v) The value of \[l=(n-1)\] always. Where 'n' is the number of principle shell.
| (vi) Value of l | = | 0 | 1 | 2 | 3?..(n-1) |
| Name of subshell | = | s | p | d | f |
| Shape of subshell | = | Spherical | Dumbbell | Double dumbbell | Complex. |
(vii) It represent the orbital angular momentum. Which is equal to \[\frac{h}{2\pi }\sqrt{l(l+1)}\]
(viii) The maximum number of electrons in subshell \[=2(2l+1)\]
\[s-\text{subshell}\to 2\,\text{electrons}\] \[d-\text{subshell}\to 10\,\text{electrons}\]
\[p-\text{subshell}\to \text{6}\,\text{electrons}\] \[f-\text{subshell}\to 14\,\text{electrons}\text{.}\]
(ix) For a given value of 'n' the total values of 'l' is always equal to the value of 'n'.
(3) Magnetic quantum number (m)
(i) It was proposed by Zeeman and denoted by 'm'.
(ii) It gives the number of permitted orientation of subshells.
(iii) The value of m varies from ?l to +l through zero.
(iv) It tells about the splitting of spectral lines in the magnetic field i.e. this quantum number proves the Zeeman effect.
(v) For a given value of 'n' the total value of 'm' is equal to \[{{n}^{2}}.\]
(vi) For a given value of 'l' the total value of 'm' is equal to\[(2l+1).\]
(vii) Degenerate orbitals : Orbitals having the same energy are known as degenerate orbitals. e.g. for p subshell \[{{p}_{x}}\,\,{{p}_{y}}\,\,{{p}_{z}}\]
(viii) The number of degenerate orbitals of s subshell =0.
(4) Spin quantum numbers (s)
(i) It was proposed by Goldshmidt & Ulen Back and denoted by the symbol of 's'.
(ii) The value of \['s'\ \,\text{is }+\text{1/2}\,\ \text{and-1/2,}\]which signifies the spin or rotation or direction of electron on it's axis during movement.
(iii) The spin may be clockwise or anticlockwise.
(iv) It represents the value of spin angular momentum is equal to \[\frac{h}{2\pi }\sqrt{s(s+1)}.\]
(v) Maximum spin of an atom \[=1/2\times \]number of unpaired electron.
(vi) This quantum number is not the result of solution of schrodinger equation as solved for H-atom. Distribution of electrons among the quantum levels
| n | l | m | Designation of orbitals | Number of Orbitals in the subshell |
| 1 | 0 | 0 | 1s | 1 |
| 2 | 0 | 0 | 2s | 1 |
| 2 | 1 | -1, 0, +1 | 2p | 3 |
| 3 | 0 | 0 | 3s | 1 |
| 3 | 1 | -1, 0, +1 | 3p | 3 |
| 3 | 2 | -2, ?1, 0, +1, +2 | 3d | 5 |
| 4 | 0 | 0 | 4s | 1 |
| 4 | 1 | -1, 0, +1 | 4p | 3 |
| 4 | 2 | -2, -1, 0, +1, +2 | 4d | 5 |
| 4 | 3 | -3, -2, -1, 0, +1, +2, +3 | 4f | 7 |
Shape of orbitals
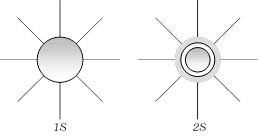
(1) Shape of ?s? orbital
(i) For 's' orbital l=0 & m=0 so 's' orbital have only one unidirectional orientation i.e. the probability of finding the electrons is same in all directions.
(ii) The size and energy of 's' orbital with increasing 'n' will be \[1s<2s<3s<4s.\]
(iii) s-orbitals known as radial node or modal surface. But there is no radial node for 1s orbital since it is starting from the nucleus.
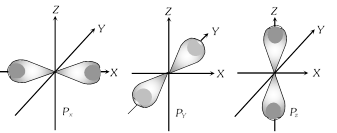
(2) Shape of 'p' orbitals
(i) For 'p' orbital l=1, & m=+1,0,-1 means there are three 'p' orbitals, which is symbolised as \[{{p}_{x}},{{p}_{y}},{{p}_{z}}.\]
(ii) Shape of 'p' orbital is dumb bell in which the two lobes on opposite side separated by the nodal plane.
(iii) p-orbital has directional properties.
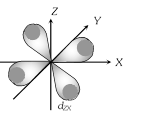
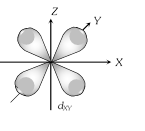
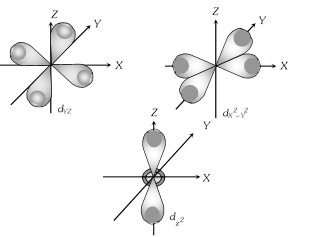
(3) Shape of 'd' orbital
(i) For the 'd' orbital l =2 then the values of 'm' are -2, -1, 0, +1, +2. It shows that the 'd' orbitals has five orbitals as \[{{d}_{xy}},{{d}_{yz}},{{d}_{zx}},\,{{d}_{{{x}^{2}}-{{y}^{2}}}},{{d}_{{{z}^{2}}.}}\]
(ii) Each 'd' orbital identical in shape, size and energy.
(iii) The shape of d orbital is double dumb bell .
(iv) It has directional properties.
(4) Shape of 'f' orbital
(i) For the 'f' orbital l=3 then the values of 'm' are -3, -2, -1,0,+1,+2,+3. It shows that the 'f' orbitals have seven orientation as \[{{f}_{x({{x}^{2}}-{{y}^{2}})}},{{f}_{y({{x}^{2}}-{{y}^{2}})}},{{f}_{z({{x}^{2}}-{{y}^{2}}),}}{{f}_{xyz}},{{f}_{{{z}^{3}}}},{{f}_{y{{z}^{2}}}}\,\text{and}\,{{f}_{x{{z}^{2}}}}.\]
(ii) The 'f' orbital is complicated in shape.
You need to login to perform this action.
You will be redirected in
3 sec
