Distinction Between Alkanes, Alkenes And Alkynes
Category : JEE Main & Advanced
| Property | Alkane (Ethane) | Alkene (Ethene) | Alkyne (Ethyne) |
| Molecular formula | \[{{C}_{n}}{{H}_{2n+2}}({{C}_{2}}{{H}_{6}})\] | \[{{C}_{n}}{{H}_{2n}}({{C}_{2}}{{H}_{4}})\] | \[{{C}_{n}}{{H}_{2n2}}({{C}_{2}}{{H}_{2}})\] |
| Nature | Saturated | Unsaturated | Unsaturated |
|
Single bond between carbon atoms. Each carbon atom is \[s{{p}^{3}}-\]hybridized
Bond length \[1.54\,\overset{o}{\mathop{A}}\,\]
Bond energy : \[83\text{ }Kcal\text{ }mo{{l}^{-1}}\] |
Double bond between two carbon atoms. Both carbon atoms are \[s{{p}^{2}}-\]hybridized
\[1.34\overset{o}{\mathop{A}}\,\] \[146\,\,Kcal\,\,mo{{l}^{-1}}\] |
Triple bond between two carbon atoms both carbon atoms are sp-hybridized \[-C\equiv C-\] \[1.20\,\,\overset{o}{\mathop{A}}\,\] \[200\,\,Kcal\,\,mo{{l}^{-1}}\] |
|
| Burning |
Burns with nonluminous flame \[{{C}_{2}}{{H}_{6}}+7/2{{O}_{2}}\to \]\[2C{{O}_{2}}+3{{H}_{2}}O\] |
Burns with luminous flame \[{{C}_{2}}{{H}_{4}}+3{{O}_{2}}\to \]\[2C{{O}_{2}}+2{{H}_{2}}O\] |
Burns with smoky flame \[{{C}_{2}}{{H}_{2}}+5/2{{O}_{2}}\to \]\[2C{{O}_{2}}+{{H}_{2}}O\] |
|
Reaction with \[{{H}_{2}}\] |
– |
Forms alkane \[{{C}_{n}}{{H}_{2n}}+\text{ }{{H}_{2}}\xrightarrow[{{300}^{o}}C]{Ni}\]\[\underset{{{C}_{n}}{{H}_{2n+2}}}{\mathop{{{C}_{n}}{{H}_{2n+2}}}}\,\] \[{{C}_{2}}{{H}_{4}}+\text{ }{{H}_{2}}\to \]\[{{C}_{2}}{{H}_{6}}\] |
Forms alkene and alkane \[{{C}_{n}}{{H}_{2n}}+\text{ }{{H}_{2}}\xrightarrow[{{300}^{o}}C]{Ni}\]\[~\underset{Alkane}{\mathop{{{C}_{n}}{{H}_{2n+2}}}}\,\] \[{{C}_{n}}{{H}_{2n2}}+\text{ }{{H}_{2}}~\xrightarrow[{{300}^{o}}C]{Ni}\]\[\underset{Alkene}{\mathop{{{C}_{n}}{{H}_{2n}}}}\,\] |
|
Reation with conc. \[{{H}_{2}}S{{O}_{4}}\] and hydrolysis |
– |
Addition \[{{C}_{2}}{{H}_{4}}+{{H}_{2}}S{{O}_{4}}\xrightarrow[{}]{{}}\] \[{{C}_{2}}{{H}_{5}}HS{{O}_{4}}\xrightarrow{{{H}_{2}}O}\] \[\underset{Alcohol}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,\] |
Addition \[{{C}_{2}}{{H}_{2}}\to C{{H}_{3}}CH{{(HS{{O}_{4}})}_{2}}\]\[\xrightarrow{{{H}_{2}}O}\]\[\underset{Aldehyde}{\mathop{C{{H}_{3}}CHO}}\,\] |
|
\[B{{r}_{2}}/CC{{l}_{4}}\] |
– |
Decolourises Dibromo derivative, \[{{C}_{2}}{{H}_{4}}+B{{r}_{2}}\to \]\[{{C}_{2}}{{H}_{4}}B{{r}_{2}}\] |
Decolourises Tetrabromo derivative, \[{{C}_{2}}{{H}_{2}}B{{r}_{4}}\] |
|
Baeyer’s reagent (Alk. \[KMn{{O}_{4}}\]) |
– |
Decolourises Glycol is formed \[\begin{array}{*{35}{l}} C{{H}_{2}} \\|\,| \\C{{H}_{2}} \\\end{array}+{{H}_{2}}O+O\to \begin{array}{*{35}{l}}C{{H}_{2}}OH \\ | \\ C{{H}_{2}}OH \\\end{array}\] |
Decolourises Oxalic acid is formed \[\begin{array}{*{35}{l}}CH \\|\,|\,| \\CH \\\end{array}+4O\to \begin{array}{*{35}{l}} COOH \\| \\COOH \\\end{array}\] |
|
Ammonical \[C{{u}_{2}}C{{l}_{2}}\] |
– |
– |
Red precipitate \[\begin{array}{*{35}{l}}CH \\|\,|\,| \\CH \\\end{array}+C{{u}_{2}}C{{l}_{2}}+2N{{H}_{4}}OH\to \underset{\text{(Red)}}{\mathop{\begin{array}{*{35}{l}}CCu \\|\,|\,| \\CCu \\\end{array}}}\,\]\[+\text{ }2N{{H}_{4}}Cl\text{ }+\text{ }2{{H}_{2}}O\] |
|
Ammonical silver nitrate |
– |
– |
\[\begin{array}{*{35}{l}}CH \\|\,|\,| \\CH \\\end{array}+2AgN{{O}_{3}}+2N{{H}_{4}}OH\to \begin{array}{*{35}{l}}C-Ag \\|\,|\,| \\C-Ag \\\end{array}\] \[+\text{ }2N{{H}_{4}}Cl\text{ }+\text{ }2{{H}_{2}}O\] |
Cycloalkane
(1) Methods of preparation
(i) From dihalogen compounds (Freund reaction):
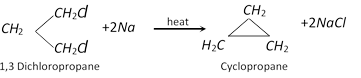
(ii) From alkenes :
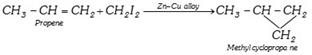
(iii) From Aromatic compounds

(2) Physical properties
(i) First two members are gases, next three members are liquids and higher ones are solids.
(ii) They are insoluble in water but soluble in alcohol and ether.
(iii) Their boiling points show a gradual increase with increase of molecular mass. Their boiling points are higher than those of isomeric alkenes or corresponding alkanes.
(iv) Their density increase gradually with increase of molecular mass.
(3) Chemical properties : Cycloalkanes behave both like alkenes and alkanes in their chemical properties. All cycloalkanes undergo substitution reaction with halogen in the presence of light (like alkane). All cycloalkane (lower members) undergo addition reaction (ex. Addition of \[{{H}_{2}},\,HX,\,{{X}_{2}}\]). Further the tendency of forming addition compounds decreases with increase in size of ring cyclopropane > Cyclobutane > Cyclopentane. Relative ring opening of ring is explained by Baeyer strain theory.
(i) Addition in spiro cycloalkane : If two cycloalkane fused with one another then addition take place in small ring
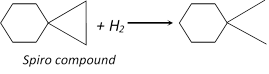
Because small ring is more unstable than large ring
Higher cycloalkanes do not give addition due to more stability.
(ii) Free radical substitution with \[C{{l}_{2}}\]
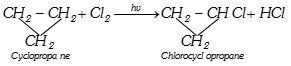
(iii) Addition reaction
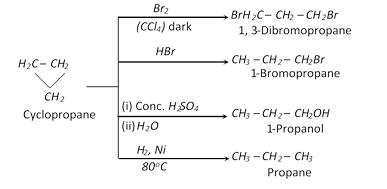
(iv) Oxidation
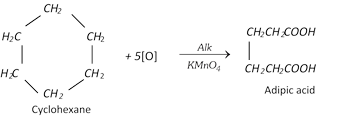
You need to login to perform this action.
You will be redirected in
3 sec
