Benzene \[\left( {{\mathbf{C}}_{\mathbf{6}}}{{\mathbf{H}}_{\mathbf{6}}} \right)\]
Category : JEE Main & Advanced
Benzene is the first member of arenes. It was first discovered by Faraday (1825) from whale oil. Mitscherllich (1833) obtained it by distillating benzoic acid with lime. Hofmann (1845) obtained it from coal tar, which is still a commercial source of benzene.
(1) Structure of benzene : Benzene has a special structure, which is although unsaturated even then it generally behave as a saturated compound.
(i) Kekule's structure : According to Kekule, in benzene 6-carbon atoms placed at corner of hexagon and bonded with hydrogen and double bond present at alternate position.
(a) Evidence in favour of Kekule's structure
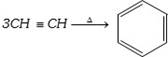
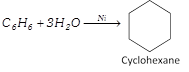
(b) Objections against Kekule's formula
Kekule explained this objection by proposing that double bonds in benzene ring were continuously oscillating between two adjacent positions.

(2) Methods of preparation of benzene
(i) Laboratory method :
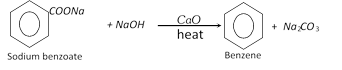
(ii) From benzene derivatives
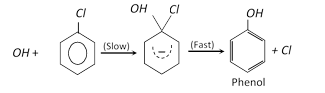
(a) From phenol :
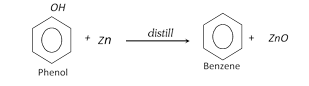
(b) From chlorobenzene :
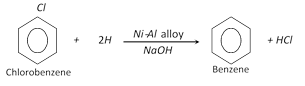
(c) By first preparing grignard reagent of chlorobenzene and then hydrolysed
\[\underset{\text{Chlorobenzene}}{\mathop{{{C}_{6}}{{H}_{5}}Cl}}\,\underset{\text{dry}\,\text{ether}}{\mathop{\xrightarrow{Mg}}}\,\underset{\text{chloride}}{\mathop{\underset{\text{Phenylmagnesium}}{\mathop{{{C}_{6}}{{H}_{5}}MgCl}}\,}}\,\xrightarrow{{{H}_{2}}O}\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,+Mg\ \ \ \ \begin{matrix}OH \\Cl \\\end{matrix}\]
(d) From benzene sulphonic acid :
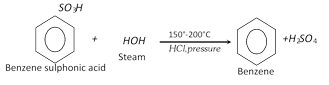
(e) From benzene diazonium chloride :
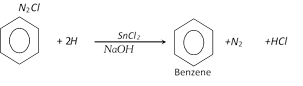
(f) From acetylene :
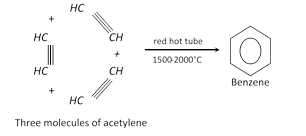
(g) Aromatisation : \[\underset{n-Hexane}{\mathop{{{C}_{6}}{{H}_{14}}}}\,\underset{\text{at high pressure}}{\mathop{\underset{500{}^\circ C}{\mathop{\xrightarrow{C{{r}_{2}}{{O}_{3}}/A{{l}_{2}}{{O}_{3}}}}}\,}}\,\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,+4{{H}_{2}}\]
(3) Properties of benzene
(i) Physical properties
(a) Benzene is a colourless, mobile and volatile liquid. It's boiling point is \[{{80}^{o}}C\] and freezing point is \[{{5.5}^{o}}C\]. It has characteristic odour.
(b) It is highly inflammable and burns with sooty flame.
(c) It is lighter than water. It's specific gravity at 20°C is 0.8788.
(d) It is immiscible with water but miscible with organic solvents such as alcohol and ether.
(e) Benzene itself is a good solvent. Fats, resins, rubber, etc. dissolve in it.
(f) It is a non-polar compound and its dipole moment is zero
(g) It is an extremely poisonous substance. Inhalation of vapours or absorption through skin has a toxic effect.
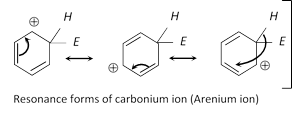
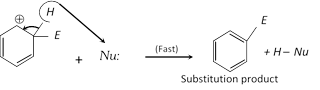
(ii) Chemical properties : Due to the presence of p electron clouds above and below the plane benzene ring, the ring serves as a source of electrons and is easily attacked by electrophiles (Electron loving reagents). Hence electrophilic substitution reaction are the characteristic reactions of aromatic compounds.
Substitution reactions in benzene are prefered rather than addition are due to the fact that in the former reactions resonance stabilised benzene ring system is retained while the addition reactions lead to the destruction of benzene ring. Principal reactions of benzene can be studied under three heads,
(a) Addition reactions
(b) Substitution reactions
(c) Oxidation reactions
(a) Addition reactions : In which benzene behaves like unsaturated hydrocarbon.
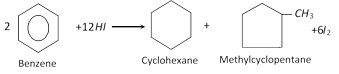
Addition of hydrogen : Benzene reacts with hydrogen in the presence of nickel (or platinum) as catalyst at \[150{}^\circ C\] under pressure to form cyclohexane.
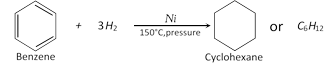
Addition of halogen :
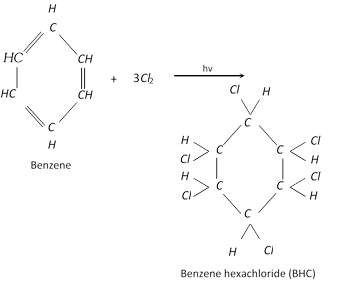
Addition of ozone :
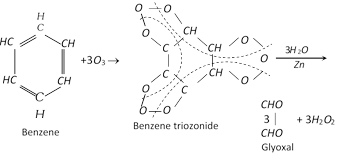
(b) Substitution reactions :
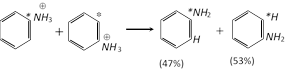
Nucleophilic substitution :
Unimolecular : Mostly uncommon in aromatic substitution, there is only one example which obtain in benzene diazonium dichloride.
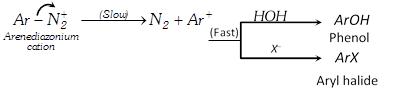
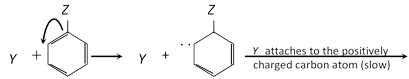
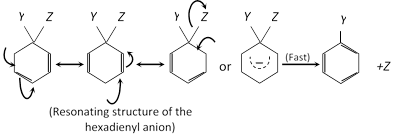
Example :
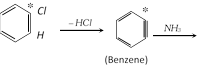

| Electrophile \[({{E}^{\oplus }})\] | Name | Source | Name of substitution reaction |
| \[C{{l}^{+}}\] | Chloronium | \[C{{l}_{2}}+AlC{{l}_{3}}\] or \[FeC{{l}_{3}}\] | Chlorination |
| \[B{{r}^{+}}\] | Bromonium | \[B{{r}_{2}}+AlB{{r}_{3}}\] or \[FeB{{r}_{3}}\] | Bromination |
| \[NO_{2}^{+}\] | Nitronium | \[HN{{O}_{3}}+{{H}_{2}}S{{O}_{4}}\] | Nitration |
| \[S{{O}_{3}}\] | Sulphur trioxide | Conc. \[{{H}_{2}}S{{O}_{4}}\], Fuming sulphuric acid | Sulphonation |
| \[{{R}^{+}}\] | Alkyl carbonium | \[RX+Al{{X}_{3}}\,(X=Cl\]or \[Br),\,ROH+{{H}^{+}}\] | Friedel-Craft's (Alkylation) |
| \[R-\overset{+}{\mathop{C}}\,=O\] | Acyl carbonium | \[RCOCl+AlC{{l}_{3}}\] | Friedel-Craft's (Acylation) |
\[{{(C{{H}_{3}})}_{3}}COOC-{{(C{{H}_{3}})}_{3}}\xrightarrow{\text{heat}}2{{(C{{H}_{3}})}_{3}}C\overset{\cdot }{\mathop{O}}\,\xrightarrow{{}}\overset{\cdot \,\,\,}{\mathop{2C{{H}_{3}}}}\,+2C{{H}_{3}}COC{{H}_{3}}\]
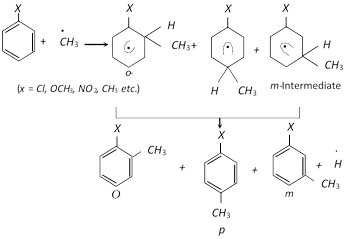
The mechanism of chlorination of benzene at high temperature is similar to that of the free radical aliphatic substitution
\[C{{l}_{2}}\xrightarrow{{}}\overset{.}{\mathop{Cl}}\,\,+\overset{.}{\mathop{Cl}}\,\] (Chain initiation)
\[{{C}_{6}}{{H}_{6}}+\overset{.}{\mathop{Cl}}\,\xrightarrow{{}}\overset{.\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}}}\,+HCl\] (H- abstraction)
\[\overset{.\,\,\,\,\,\,\,\,}{\mathop{{{C}_{6}}{{H}_{5}}}}\,+C{{l}_{2}}\xrightarrow{{}}{{C}_{6}}{{H}_{5}}Cl+\overset{.}{\mathop{Cl}}\,\] (Chain propagation)
(c) Oxidation : \[2{{C}_{6}}{{H}_{6}}+15{{O}_{2}}\xrightarrow{{}}12C{{O}_{2}}+6{{H}_{2}}O\] \[\Delta H=\]6530 kJ/mole
When vapours of benzene and air are passed over vanadium pentoxide at \[450-{{500}^{o}}C\], maleic anhydride is obtained.
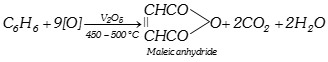
(d) Reduction :
(iii) Uses : (a) In dry cleaning (b) As a motor fuel when mixed with petrol. (c) As a solvent. (d) In the manufacture of gammexane (As insecticide). (e) In the preparation of nitrobenzene, chlorobenzene, benzene sulphonic acid, aniline, styrene, etc. Many of these are employed for making dyes, drugs, plastics, insecticides, etc.
You need to login to perform this action.
You will be redirected in
3 sec
