Optical Isomerism
Category : JEE Main & Advanced
(1) Compounds having similar physical and chemical properties but they have the ability to rotate the plane of polarised light either to the right (Clockwise) or to the left (Anticlockwise) are termed as optically active or optical isomers and the property is called optical activity or optical isomerism.
The optical activity was first observed in organic substances like quartz, rock-crystals and crystals of potassium chlorate \[(KCl{{O}_{3}})\], potassium bromate \[(KBr{{O}_{3}})\] and sodium periodate \[(NaI{{O}_{4}})\].
(2) Measurement of optical activity : The measurement of optical activity is done in terms of specific rotation which is defined as the rotation produced by a solution of length of 10 centimetres (One decimetre) and unit concentration (1 g/mL) for the given wavelength of the light at the given temperature.
Specific rotation, \[\left[ \alpha \right]_{\text{wavelength}}^{t{}^\circ C}=\frac{{{\alpha }_{obs}}}{l\times C}\]
Where \[{{\alpha }_{obs}}\] is the rotation observed, \[l\] is the length of the solution in decimeters and C is the number of grams in 1mL of solution. The specific rotation of the sucrose at \[{{20}^{o}}C\] using sodium light (D-line, \[\lambda =5893\overset{o}{\mathop{A}}\,\]) is \[+{{66.5}^{o}}C\] and is denoted as: \[\left[ \alpha \right]_{D}^{20{}^\circ C}=+66.5{}^\circ C(C=0.02\,g/mL\]water) + sign indicates the rotation in clockwise direction.
(3) On the basis of the study of optical activity, the various organic compounds were divided into four types :
(i) The optical isomer which rotates the plane of the polarised light to the right (Clockwise) is known as dextrorotatory isomer (Latin: dextro = right) or d-form or indicated by sign.
(ii) The optical isomer which rotates the plane of the polarised light to the left (Anticlockwise) is known as laevorotatory isomer (Latin; laevo = left) or form or indicated by sign.
(iii) The optical powers of the above two isomers are equal in magnitude but opposite in sign. An equimolar mixture of the two forms, therefore, will be optically inactive due to external compensation. This mixture is termed as racemic mixture or dl-form ormixture.
(iv) Optical isomer with a plane of symmetry is called meso form. It is optically inactive due to internal compensation, i.e., the rotation caused by upper half part of molecule is neutralised by lower half part of molecule.
(4) Chirality, (i) Definition : A molecule (or an object) is said to be chiral or dissymmetric, if it is does not possess any element of symmetry and not superimposable on its mirror image and this property of the molecule to show non-superimposability is called chirality.
On the other hand, a molecule (or an object) which is superimposable on its mirror image is called achiral (non-dissymmetric or symmetric).
To understand the term chiral and achiral let us consider the alphabet letters ‘P’ and ‘A’ whereas ‘P’ is chiral, ‘A’ is achiral as shown in fig.

(ii) Elements of symmetry : There are three elements of symmetry,
(a) Plane of symmetry : It may be defined as a plane which divides a molecule in two equal parts that are related to each other as an object and mirror image. e.g.,
(b) Centre of symmetry : It may be defined as a point in the molecule through which if a line is drawn in one direction and extended to equal distance in opposite direction, it meets another similar group or atom, eg.
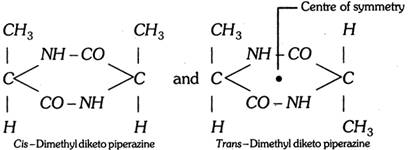
Since trans form contains a centre of symmetry, it is optically inactive.
(c) Alternating axis of symmetry : A molecule is said to possess an alternating axis of symmetry if an oriention indistinguishable from the original is obtained when molecule is rotated \[Q\] degree around an axis passing through the molecule and the rotated molecule is reflected in a mirror that is perpendicular to the axis of rotation in step (I).
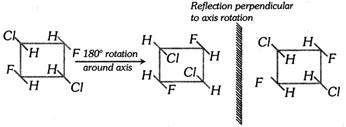
(iii) Symmetric, Asymmetric and Dissymmetric molecules
(a) Symmetric molecules : If any symmetry is present in the molecule then molecule will be symmetric molecule.
(b) Dissymmetric molecules : Molecule will be a dissymmetric molecule if it has no plane of symmetry, no centre of symmetry and no alternating axis of symmetry.
(c) Asymmetric molecules : Dissymmetric molecule having at least one asymmetric carbon is known as asymmetric molecule. All asymmetric molecules are also dissymmetric molecules but the reverse is not necessarily true.
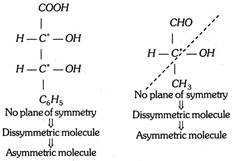
(iv) Chiral or asymmetric carbon atom : A carbon bonded to four different groups is called a chiral carbon or a chirality centre. The chirality centre is indicated by asterisk. e.g.,
\[d-\overset{a}{\mathop{\overset{|}{\mathop{\underset{c}{\mathop{\underset{|}{\mathop{{{C}^{*}}}}\,}}\,}}\,}}\,-b\] \[\underset{\text{Lactic acid}}{\mathop{HO-\underset{COOH}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{C{{H}_{3}}\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{{{C}^{*}}-H}}\,}}\,}}\,}}\,}}\,\]
\[\begin{matrix} D \\ | \\ H{{C}^{*}}T \\ | \\ Br \\ \end{matrix}\] \[\underset{\text{Lactic}\,\text{acid}}{\mathop{\begin{matrix} {{H}_{1}} \\ |\,\, \\ C{{l}^{35}}{{C}^{*}}C{{l}^{37}} \\ | \\ {{H}_{2}} \\ \end{matrix}}}\,\]
\[-C{{H}_{3}},\,\,\,-C{{H}_{2}}OH,\,\,\,-CH{{X}_{2}},\,\,-CHO,\,\,\,-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-Z\]
\[(HOOC-C{{H}_{2}}-CHOH-COOH)\] show optical isomerism.
(5) Calculation of number of optical isomers
(i) If molecule is not divisible into two identical halves and molecule has \[n\] asymmetric carbon atoms then
Number of optically active forms \[={{2}^{n}}=a\]
Number of enantiomeric pair \[=a/2\]
Number of racemic mixture \[=a/2\]
Number of meso form \[=0\]
(ii) If molecule is divisible into two identical halves, then the number of configurational isomers depends on the number of asymmetric carbon atoms.
Case I : When compound has even number of carbon atoms, i.e., \[n=2,\,4,\,8,\,10,\,12,\,.....\]:
(i) Number of optically by active forms \[=a={{2}^{n-1}}\]
(ii) Number of enantiomeric pairs \[=a/2\]
(iii) Number of racemic mixture \[=a/2\]
(iv) Number of meso forms \[=m={{2}^{(n/2)-1}}\]
(v) Total number of configurational isomers \[=a+m\]
Case II : When compound has odd number of carbon atoms, i.e., \[n=3,\,5,\,7,\,9,\,11,\,......\]:
(i) Number of optically active forms \[=a={{2}^{n-1}}-{{2}^{(n-1)/2}}\]
(ii) Number of enantiomeric pairs \[=a/2\]
(iii) Number of racemic mixutre \[=a/2\]
(iv) Number of meso forms \[=m={{2}^{(n-1)/2}}\]
(v) Total number of configurational isomers \[=a+m\]
(6) Optical activity of compounds containing one asymmetric carbon
Examples :
\[\underset{\text{Lactic acid}}{\mathop{C{{H}_{3}}-C\overset{*\,\,\,\,\,\,\,\,\,}{\mathop{HOH}}\,-COOH}}\,\]; \[C{{H}_{3}}-C\overset{*\ \ \ \ \ \ }{\mathop{HOH}}\,-CHO\]
\[\underset{\text{Glyceraldehyde}}{\mathop{C{{H}_{2}}OH-C\overset{*\ \ \ \ \ \ }{\mathop{HOH}}\,-CHO}}\,\] ; \[\underset{\text{1-chloro-1-phenylethane}}{\mathop{{{C}_{6}}{{H}_{5}}-C\overset{*\ \ \ \ }{\mathop{HCl}}\,-C{{H}_{3}}}}\,\]
Any molecule having one asymmetric carbon atom exists in two configurational isomers which are nonsuperimposible mirror images.
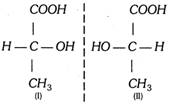
(I) and (II) have the same molecular formula, the same structure but different configurations, hence (I) and (II) are known as configurational isomers. (I) and (II) are nonsuperimposable mirror images, hence (I) and (II) are optical isomers. Configurational isomers which are nonsuperimposable mirror images are known as enantiomers. Thus (I) and (II) are enantiomers. Pair of (I) and (II) is known as enantiomeric pair.
(i) Properties of Enantiomers : All chemical and physical properties of enantiomers are same except two physical properties.
Mode of rotation : One enantiomer rotates light to the right and the other by an equal magnitude to the left direction.
(ii) Racemic Mixture : An equimolar mixture of two enantiomers is called a racemic mixture (or racemate, \[\pm \] form, (dl) form or racemic modification). Such a mixture is optically inactive because the two enantiomers rotate the plane polarised light equally in opposite directions and cancel each other’s rotation. This phenomenon is called external compensation.
\[\Rightarrow \]Racemic mixture can be separated into \[(+)\] and \[(-)\]forms. The separation is known as resolution.
\[\Rightarrow \]The conversion of \[(+)\] or \[(-)\] form of the compound into a racemic mixture is called racemisation. It can be caused by heat, light or by chemical reagents.
\[\Rightarrow \]Racemic mixture is designated as being \[(\pm )\] or \[(dl)\].
(7) Optical activity of compounds containing two asymmetric carbon
Case I : When molecule is not divisible into two identical halves.
The number of optical isomers possible in this case is four \[(a={{2}^{2}}=4)\]. Further there will be two pairs of enantiomers and two racemic modifications. In practice also it is found to be so.
Configurational isomers which are not mirror images are known as diastereomers.
Properties of Diastereomers : Diastereomers have different physical properties, e.g., melting and boiling points, refractive indices, solubilities in different solvents, crystalline structures and specific rotations. Because of differences in solubility they often can be separated from each other by fractional crystallisation; because of slight differences in molecular shape and polarity, they often can be separated by chromatography.
Diastereomers have different chemical properties towards both chiral and achiral reagents. Neither any two diastereomers nor their transition states are mirror images of each other and so will not necessarily have the same energies. However, since the diastereomers have the same functional groups, their chemical properties are not very dissimilar.
Case II : When molecule is divisible into two identical halves.
Number of optical isomers \[=a={{2}^{2-1}}=2\]
Number of meso forms \[=m={{2}^{0}}=1\]
Total number of configurational isomers \[=3\]
(8) Optical activity in compounds containing no assymmetric carbon : Although the largest number of known optically active compounds are optically active due to the presence of chiral carbon atom, some compounds are also known which do not possess any chiral carbon atom, but on the whole their molecules are chiral (such molecules were earlierly called dissymmetric); hence they are optically active. Various types of compounds belonging to this group are allenes, alkylidene cycloalkanes, spiro compounds (spirans) and properly substituted biphenyls.
(i) Allenes : Allenes are the organic compounds of the following general formulae.
![]()
Allenes exhibit optical isomerism provided the two groups attached to each terminal carbon atom are different, i.e.,
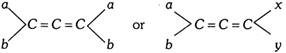
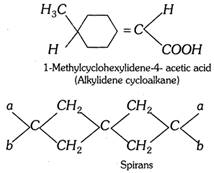
(ii) Alkylidene cycloalkanes and spiro compounds : When one or both of the double bonds in allenes are replaced by one and two rings, the resulting systems are respectively known as alkylidene cycloalkanes and spirans.
(iii) Biphenyls : Suitably substituted diphenyl compounds are also devoid of individual chiral carbon atom, but the molecules are chiral due to restricted rotation around the single bond between the two benzene nuclei and hence they must exist in two non-superimposable mirror images of each other. Such types of stereoisomerism which is due to restricted rotation about single bond, is known as atropisomerism and the stereoisomers are known and atropisomers. Examples
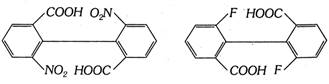
The above discussion leads to the conclusion that the essential condition for optical isomerism is the molecular disymmetry or molecular chirality and not the mere presence of a chiral centre. However, it may be noted that the molecules having only one chiral centre are always chiral and exhibit optical isomerism.
(9) Fischer projection formulae : The arrangement of the atoms or groups in space that characterises a stereoisomer is called its configuration.
Emil Fischer (1891) provided an easy method to represent the three dimensional formulae of various organic molecules on paper. Fischer projection is, thus, a planar representation of the three dimensional structure.
By convention, the following points are followed in writing the Fischer formula.
(i) The carbon chain of the compound is arranged vertically, with the most oxidised carbon at the top.
(ii) The asymmetric carbon atom is in the paper plane and is represented at the interaction of crossed lines.
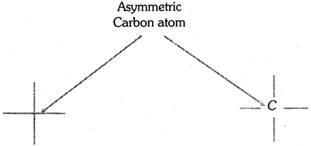
(iii) Vertical lines are used to represent bonds going away from the observer, i.e., groups attached to the vertical lines are understood to be present behind the plane of the paper.
(iv) Horizontal lines represent bonds coming towards the observer, i.e., groups attached to the horizontal lines are understood to be present above the plane of the paper.
(10) Name of optical isomers : Following three nomenclatures are used for optically active compounds,
(i) D,L. System of nomenclature : This nomenclature is mainly used in sugar chemistry or optically active polyhydroxy carbonyl compounds. This nomenclature was given by Emil Fischer to designate the configurations of various sugars relative to the enantiomeric \[(+)\] and \[(-)\]glucose as reference.
All sugars whose Fischer projection formula shows the OH group on the chiral carbon atom adjacent to the terminal \[C{{H}_{2}}OH\] group on the right hand side belong to the \[D\]-series. Similarly if \[OH\] is on the left hand side, then the sugars belong to the \[L\]-series.
\[\underset{D-\text{series}}{\mathop{H\underset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{COH}}\,}}\,}}\,}}\,\] \[\underset{L-\text{series}}{\mathop{HO\underset{C{{H}_{2}}OH}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CH\,\,\,}}\,}}\,}}\,}}\,\]
Examples :
\[\underset{D(+)\,\text{glyceraldehyde}}{\mathop{\underset{\text{or}}{\mathop{\underset{D(d)\,\text{glyceraldehyde}}{\mathop{\begin{matrix} \,\,\,\,\,\,\,\,\,\,\,CHO \\ \,\,\,\,\,H\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,OH \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{2}}OH \\ \end{matrix}}}\,}}\,}}\,\] \[\underset{L(-)\,\text{glyceraldehyde}}{\mathop{\underset{\text{or}}{\mathop{\underset{L(l)\,\text{glyceraldehyde}}{\mathop{\begin{matrix} \,\,\,\,\,\,\,\,CHO \\ HO\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,H\,\,\,\, \\ \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{2}}OH \\ \end{matrix}}}\,}}\,}}\,\]
\[\Rightarrow \]It must be noted that there is no relation between the sign of rotation (\[+,\,\,-\] or d, l) and the configuration (D and L) of an enantiomer.
\[\Rightarrow \]Any compound that can be prepared from, or converted into \[D(+)\] glyceraldehyde will belong to D-series and similarly any compound that can be prepared from, or converted into \[L(-)\] glyceraldehyde will belong to the L-series.
\[\Rightarrow \]This nomenclature is also used in \[\alpha \]-amino acids.
(ii) Erythro and Threo system of nomenclature : This nomenclature is used only in those compounds which have
(a) Only two chiral carbons and
(b) The following structure, \[{R}'-Cab-Cbc-{R}''\]
i.e., out of six substituents on two asymmetric carbons, at least two should be same.
When two like groups in Fischer projection formula are drawn on the same side of the vertical line, the isomer is called erythro form; if these are placed on the opposite sides, the isomer is said to be threo form.
\[\underset{Erythro\,form}{\mathop{\begin{matrix} {{R}'} \\ | \\ aCb \\ | \\ cCb \\ | \\ {{R}''} \\ \end{matrix}}}\,\] \[\underset{Erythro\,form}{\mathop{\begin{matrix} \,\,\,\,\,\,\,C{{H}_{3}} \\ | \\ HCCl \\ | \\ HCBr \\ | \\ \,\,\,\,\,\,\,\,\,\,{{C}_{6}}{{H}_{5}} \\ \end{matrix}}}\,\] \[\underset{Threo\,form}{\mathop{\begin{matrix} \,\,\,\,\,\,C{{H}_{3}} \\ | \\ HCCl \\ | \\ BrCH \\ | \\ \,\,\,\,\,\,\,\,\,\,{{C}_{6}}{{H}_{5}} \\ \end{matrix}}}\,\]
(c) R,S Nomenclature (Absolute configuration)
The order of arrangement of four groups around a chiral carbon (stereocentre) atom is called the absolute configuration around that atom. System which indicates the absolute configuration was given by three chemists R.S. Cahn, C.K. Ingold and V. Prelog. This system is known as (R) and (S) system or the Cahn-Ingold Prelog system. The letter (R) comes from the latin rectus (means right) while (S) comes from the latin sinister (means left). Any chiral carbon atom has either a (R) configuration or a (S) configuration. Therefore, one enantiomer is (R) and other is (S). A racemic mixture may be designated (R) (S), meaning a mixture of the two. (R) (S) nomenclature is assigned as follows :
Step I : By a set of sequence rules given below the atoms or groups connected to the chiral carbon are assigned a priority sequence.
Sequence Rules for Order of Priority
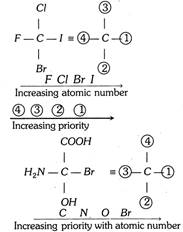
Rule 1 : If all four atoms directly attached to the chiral carbon are different, priority depends on their atomic number. The atom having highest atomic number gets the highest priority, i.e., (1). The atom with the lowest atomic number is given the lowest priority, i.e., (2), the group with next higher atomic number is given the next higher priority (3) and so on. Thus
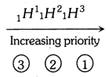
Rule 2 : If two or more than two isotopes of the same element is present, the isotope of higher atomic mass receives the higher priority.
Rule 3 : If two or more of the atoms directly bonded to the chiral carbon are identical, the atomic number of the next atoms are used for priority assignment. If these atoms also have identical atoms attached to them, priority is determined at the first point of difference along the chain. The atom that has attached to it an atom of higher priority has the higher priority.
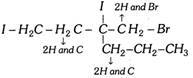
In this example the atoms connected directly to the chiral carbon are iodine and three carbons. Iodine has the highest priority. Connected, to the three carbons are 2H and Br, 2H and C and 2H and C. Bromine has the highest atomic number among C,H and Br and thus \[C{{H}_{2}}Br\] has highest priority among these three groups (i.e., priority no. 2).The remaining two carbons are still identical (C and 2H) connected to the second carbons of these groups are 2H and I and 2H and C. Iodine has highest priority among these atoms, so that \[-C{{H}_{2}}-C{{H}_{2}}-I\] is next in the priority list and \[C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\] has the last priority.
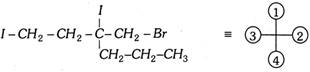
Rule 4 : If a double or a triple bond is linked to chiral centre the involved atoms are duplicated or triplicated respectively.
\[-\underset{|}{\mathop{C}}\,=O\equiv \overset{O\,\,\,\,}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-O}}\,\]; \[-C\equiv N\equiv \underset{N\,\,\,\,\,}{\overset{N\,\,\,\,}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-N}}}\,\]; \[\overset{O\,\,\,\,\,\,\,\,\,}{\mathop{-\overset{||}{\mathop{C}}\,-OH}}\,\equiv \underset{O\,\,\,\,\,\,\,\,\,}{\overset{O\,\,\,\,\,\,\,\,\,}{\mathop{-\underset{|}{\overset{|}{\mathop{C}}}\,-OH}}}\,\]
By this rule, we obtained the following priority sequence :
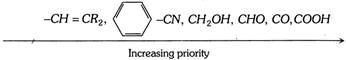
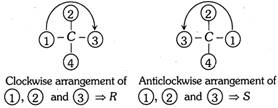
Step 2 : The molecule is then visualised so that the group of lowest priority (4) is directed away from the observes (At this position the lowest priority is at the bottom of the plane). The remaining three groups are in a plane facing the observer. If the eye travels clockwise as we look from the group of highest priority to the groups of second and third priority (i.e., \[1\to 2\to 3\]with respect to 4) the configuration is designated as R. If arrangement of groups is in anticlocwise direction, the configuration is designated as S.
For example:
Let us apply the whole sequence to bromochlorofluoro methane.
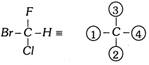
In this Fischer projection the least priority number is not at the bottom of the plane.
In such cases the Fischer projection formula of the compound is converted into another equivalent projection formula in such a manner that atom or group having the lowest priority is placed vertically downward. This may be done by two interchanges between four priority numbers. The first interchange involves the two priority numbers, one is the least priority number and other is the priority number which is present at the bottom of the plane. In the above case first interchange will takes place between 2 and 4.
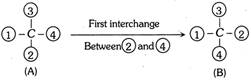
First interchange of two groups at the chiral centre inverts the configuration and this gives enantiomer of the original compound. Thus (A) and (B) are enantiomer. The second interchange involves the remaining two groups.
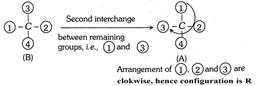
Example :
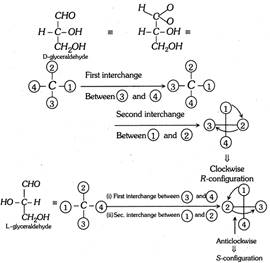
Glyceraldehyde (For example) has one asymmetric carbon, hence it has two configurational isomers (I) and (II).
\[\underset{(\text{I})}{\mathop{\underset{(R)\,\text{glyceral dehyde}}{\mathop{H-\overset{CHO\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}OH\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-OH\,\,\,\,\,\,}}\,}}\,}}\,}}\,\] \[\underset{(\text{II)}}{\mathop{\underset{(S)\,\text{glyceral dehyde}}{\mathop{HO-\overset{CHO\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}OH\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-H\,\,\,\,\,\,}}\,}}\,\,\,\,}}\,}}\,\,\,\,\,\,\,\,\,\,\]
One can draw a number other configurations for glyceraldehyde but each of them will be a repetition of either (I) or (II). In this connection it is important to note that if two projection formulae differ by an odd number of interchanges (1, 3, 5, 7, …..) of positions of groups on the chiral carbon, they are different. But if the two differ by an even number of interchanges (2, 4, 6, …..) they are identical.
For example :
\[\underset{(I)\,\,\,\,\,\,\,\,\,\,\,}{\mathop{H-\overset{CHO\,\,\,\,\,\,\,\,\,}{\mathop{\underset{\,C{{H}_{2}}OH\,\,\,\,}{\mathop{\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-OH\,\,\,\,\,}}\,}}\,}}\,\underset{\text{First interchange}}{\mathop{\xrightarrow{H,\,C{{H}_{2}}OH}}}\,\underset{\,\,\,\,(II)\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\,\,\,\,C{{H}_{2}}OH-\overset{CHO}{\mathop{\underset{\,H\,\,\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-H}}\,}}\,\underset{\text{between }OH,CHO}{\mathop{\xrightarrow{\text{Second interchange}}}}\,\,\,}}\,\]
\[\,\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,(III)}{\mathop{\,C{{H}_{2}}OH-\overset{OH\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{H\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-CHO}}\,}}\,\,}}\,\underset{\text{C}{{\text{H}}_{\text{2}}}OH,OH}{\mathop{\xrightarrow{\text{Third}\,\text{intrerchange}}}}\,\]
\[\underset{(IV)}{\mathop{HO-\overset{C{{H}_{2}}OH}{\mathop{\underset{H\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-CHO}}\,}}\,}}\,\underset{H,CHO}{\mathop{\xrightarrow{\text{Fourth interchange}}}}\,\underset{(V)}{\mathop{HO-\overset{C{{H}_{2}}OH}{\mathop{\underset{CHO\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{\underset{|}{\mathop{C}}\,}}\,-H\,\,\,\,\,\,}}\,}}\,}}\,\]
Thus (I), (III) and (V) are identical. Similarly (II) and (IV) are identical.
(11) Resolution of racemic modifications : The separation of racemic mixture into its enantiomers is known as resolution.
(12) Asymmetric synthesis and Walden inversion
(i) Asymmetric synthesis : The synthesis of an optically active compound (asymmetric) from a symmetrical molecule (having no asymmetric carbon) without resolution to form \[(+)\] or \[(-)\] isomer directly is termed asymmetric synthesis. For example the reduction of pyruvic acid \[(C{{H}_{3}}-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-COOH)\] in presence of nickel catalyst gives \[(\pm )\]lactic acid (racemic mixture). On the other hand, pyruvic acid is reduced to \[(-)\] lactic acid only by yeast.
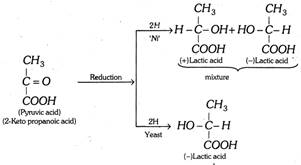
(ii) Walden inversion: The conversion of \[(+)\] form into \[(-)\]form and vice-versa is called Walden inversion. When an atom or group directly linked to an asymmetric carbon atom is replaced; the configuration of the new compound may be opposite to (inverse) that of the original, i.e.,
\[\underset{(+)\text{ Malic acid}}{\mathop{H-\overset{C{{H}_{3}}\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}COOH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-OH\,\,\,\,\,\,\,\,}}\,}}\,}}\,\xrightarrow{PC{{l}_{5}}}\underset{(-)\text{ Chloro succinic acid}}{\mathop{Cl-\overset{C{{H}_{3\,}}\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}COOH\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-H\,\,\,\,\,\,\,\,\,\,\,\,\,}}\,}}\,}}\,\xrightarrow{AgOH}\underset{(-)\,\text{Malic acid}}{\mathop{HO-\underset{C{{H}_{2}}COOH}{\mathop{\overset{\,COOH\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-H\,\,\,\,\,\,\,\,\,\,\,\,}}\,}}\,}}\,\]
\[\xrightarrow{PC{{l}_{5}}}\underset{(+)\text{ Chloro succinic acid}}{\mathop{H-\overset{COOH\,\,\,\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}COOH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-Cl\,\,\,\,\,\,\,\,\,\,\,\,}}\,}}\,}}\,\xrightarrow{AgOH}\underset{(+)\text{ Malic acid}}{\mathop{H-\overset{COOH\,\,\,\,\,\,\,\,\,}{\mathop{\underset{C{{H}_{2}}COOH}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-OH\,\,\,\,\,\,\,\,\,}}\,}}\,}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
