Mode Of Enzyme Action
Category : 11th Class
In 1913 Michaelis and Menten proposed that for a catalylic reaction to occur it is necessary that enzyme and substrate bind together to form an enzyme substrate complex.
\[\underset{(Enzyme)}{\mathop{E}}\,+\underset{(Substrate)}{\mathop{S}}\,\to \underset{(Enzyme-substrate\text{ }Complex)}{\mathop{E-S\text{ }Complex}}\,\]
\[E-S\text{ }Complex\to \underset{(Enzyme)}{\mathop{E}}\,+\underset{(\Pr oduct)}{\mathop{P}}\,\]
It is amazing that the enzyme-substrate complex breaks up into chemical products different from those, which participated in its formation (i.e., substrates). On the surface of each enzyme there are many specific sites for binding substrate molecules called active sites or catalytic sites.
There are two views regarding the mode of enzyme action :
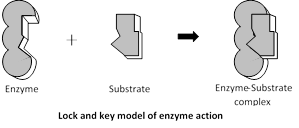
Lock and Key hypothesis : The hypothesis was put forward by Emil Fisher (1894). According to this hypothesis the enzyme and its substrate have a complementary shape. The specific substrate molecules are bound to a specific site of the enzyme molecule.
The theory can be explained easily by the fact that a particular lock can be opened by a particular key specially designed to open it. Similarly enzymes have specific sites where a particular substrate can only be attached. The lock and key model accounts for enzyme specificity.
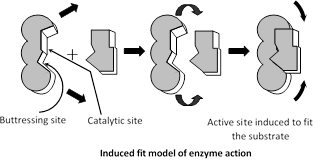
Induced fit hypothesis : This hypothesis was proposed by Daniel, E. Koshland (1959).
According to this view, active site is not rigid but static and it has two groups - buttressing group and catalytic group. Initially substrate bind to the buttressing group which induces the catalytic group to fit the substrate and catalytic group weakes the bonds of reactant or substrate by electrophilic and nucleophilic forces.
You need to login to perform this action.
You will be redirected in
3 sec
