Periodic Classification of Elements
Category : 10th Class
Periodic Classification of Elements
At present about 115 elements are known. All the elements are divided into groups such that the elements in the same group have similar properties. The periodic classification of elements helps in systematic study of elements and makes it easy to understand the properties of elements.
Dobereiners’s Triads
Johann Wolfgang Dobereiner, a German scientist, was the first to classify elements. He grouped the elements with similar chemical properties into groups of three called ‘Triads’. When elements were arranged in order of their increasing atomic mass, the atomic mass of the middle element was equal to the approximate arithmetic mean of the atomic masses of the other two elements of the triad. For example, Li, Na, K and Cl, Br, I.
Limitations of Triad Classification
Newlands’ Octaves
John Alexander Reina Newland arranged many of the known elements in the increasing order their atomic masses.
He noticed that every eighth element was similar in properties to the first element.
The eighth element after lithium is sodium. It is similar to lithium in many of its chemical properties. Similarly, the eighth element after sodium is potassium, whose properties are similar to sodium. The eighth element from fluorine is chlorine both of which are similar in their properties. The eighth element from nitrogen is phosphorus and both these elements are similar in properties.
Based on this observation, Newland stated his law of octaves. According to this law 'when elements are arranged in increasing order of their atomic mass, the eighth elements resembles the first in physical and chemical properties'. This repetition of properties of elements gave rise to a new term called periodicity. Periodicity is the recurrence of characteristic properties of elements arranged in a table, at regular intervals of a period
Advantages of the Law of Octaves
Mendeleev's Periodic Law
Later, Mendeleev arranged the sixty-three elements known at that time in the increasing order of the atomic masses, in the form of a table called the Periodic Table. Mendeleev's periodic table further classified the elements by arranging the elements with similar properties together and separating the elements with dissimilar properties from one another.
Mendeleev stated the law of chemical periodicity as: "The physical and chemical properties of elements are periodic functions of their atomic masses."
Mendeleev's periodic table contains eight vertical columns of elements called 'groups' and seven horizontal rows called 'periods.
Achievements of Mendeleev's Periodic Table
Limitation of Mendeleev's Periodic Table
Modern Periodic Law
The properties of elements are a periodic function of their atomic numbers. When the elements are arranged according to increasing atomic numbers, then the elements having same number of valence electrons occur at regular intervals (or periods).
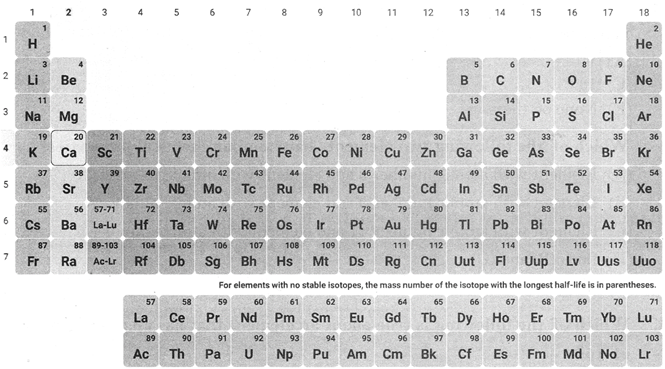
Modern Periodic Table
The modern periodic table was prepared by Bohr.
The modern periodic table has 18 vertical columns known as groups and 7 horizontal known as periods.
Periods
General Characteristics of Periods and Groups
|
Characteristics |
Periods(From left to right) |
Groups (Down the group) |
|
1.Valence Electrons |
Increases |
Number of Valence electrons in the elements remain the same |
|
2.Valency |
First increases then decreases |
All the elements in a group have the same valency |
|
3.Atomic size |
Decreases |
Increases |
|
4.Metallic character |
Decreases but non- metallic character increases |
Increases |
|
5.Electropositive character |
Decreases |
Increases |
|
6.Chemical reactivity |
First decreases then increases |
Increases |
|
7. Nature of oxides |
The basic nature of oxides decreases and the acidic nature of oxides increases |
No change in the nature of oxides of elements |
You need to login to perform this action.
You will be redirected in
3 sec
