Metals and Non- Metals
- Metals and non-metals: On the basis of properties, all the elements can be divided into two main groups: metals and non-metals.
- Sodium, Potassium, Calcium and Magnesium form positive ions. A majority of the known elements are metals. All metals are solids, except mercury which is a liquid metal.
- The most abundant metal in the earth's crust is aluminium.
- Though non-metals are small in number as compared to metals, they play a very important role in our daily life.
- The most abundant non-metal in the earth's crust is oxygen.
- Metallurgy: Metallurgy is the branch of chemistry that deals with the extraction of metals from their ores.
- Physical Processes involves crushing, grinding and concentration of the ore, gravity separation, froth floatation and electromagnetic separation.
- Chemical Processes involve roasting, calcination, reduction of metallic oxide to free metal, reduction of ore and refining of metals.
- Refining of metals: Metals obtained by the above processes contain impurities such as presence of other metals, non-metals like silicon or phosphorus, unreduced oxides and sulphides of the metal. Following processes involve refining of various metals:
(i) Liquidation: This method is used to refine metals having a low melting point, e.g., lead and tin.
(ii) Distillation: Used to refine volatile metals like mercury and zinc which contain non volatile impurities.
(iii) Oxidation: Used to refine metals containing volatile impurities, which are easily oxidised, e.g., pig iron.
(iv) Electro - refining: This is an economical and effective method for purifying metals, e.g., copper, aluminium, lead.
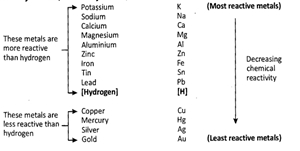
- Reactivity series (or activity series) of metals