Functional Groups
Category : 10th Class
A group of compound which determines the property of the hydrocarbon is called functional group. Particular types of reactions are associated with the functional groups with different structural attachments, resulting to names associated with such compounds.
The carbonyl group is the functional group involved with several of the hydrocarbon derivatives shown above. The carboxyl group is present in amino acids and carboxylic acids.
In chemistry, an alcohol is any organic compound in which a hydroxyl functional group (-OH) is bound to a carbon atom, usually connected to other carbon or hydrogen atoms. An important class are the simple acyclic alcohols, the general formula for which is\[{{C}_{n}}{{H}_{2n+1}}OH\] . Of those, ethanol \[({{C}_{2}}{{H}_{5}}OH)\] is the type of alcohol found in alcoholic beverages, and in common speech the word alcohol refers specifically to ethanol.
![]()
The most commonly used alcohol is ethanol, \[{{\mathbf{C}}_{\mathbf{2}}}{{\mathbf{H}}_{\mathbf{5}}}\mathbf{OH}\]. Ethanol has been produced and consumed by humans for millions of years, in the form of fermented and distilled alcoholic beverages. It is a clear flammable liquid that boils at 78.4 °C, which is used as an industrial solvent, car fuel, and raw material in the
chemical industry. In many countries, because of legal and tax restrictions on alcohol consumption, ethanol destined for other uses often contains additives that make it unpalatable poisonous. Ethanol in this form is known generally as denatured alcohol. When methanol is used, it may be referred to as methylated spirits.
Methanol \[{{(C{{H}_{3}}OH)}^{-}}\] Wood alcohol, Ethanol \[{{({{C}_{2}}{{H}_{5}}OH)}^{-}}\] Grain alcohol, IsopropyI alcohol \[{{({{C}_{3}}{{H}_{7}}OH)}^{-}}\] Rubbing alcohol, Pentanol \[{{({{C}_{5}}{{H}_{11}}OH)}^{-}}\] Amyl alcohol, Hexadecan-1-ol (C^H^OH)- Cetyl alcohol, Polyhydric alcohols \[{{C}_{2}}{{H}_{4}}{{(OH)}_{2}}^{-}\] Ethane-1 ,2-diol, Ethylene glycol \[{{C}_{3}}{{H}_{5}}{{(OH)}_{3}}\] Propane-1,2,3-triol, Glycerin \[{{C}_{4}}{{H}_{6}}{{(OH)}_{4}}^{-}\] Butane-1,2,3,4-tetraol, Erythritol \[{{C}_{5}}{{H}_{7}}{{(OH)}_{5}}\]-Pentane- 1, 2, 3, 4, 5 - pentol.
\[\underset{methanol}{\mathop{HO-C{{H}_{3}}}}\,\] \[\underset{ethanol}{\mathop{HO-C{{H}_{2}}-C{{H}_{3}}}}\,\] \[\underset{1-propanol}{\mathop{HO-C{{H}_{2}}-CH-C{{H}_{3}}}}\,\]
\[\underset{\text{1-octanol}}{\mathop{HO-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}}}\,\]
\[\underset{\text{2-methyl-1-butanol}}{\mathop{HO-C{{H}_{2}}-\underset{\begin{smallmatrix}
| \\
C{{H}_{3}}
\end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}-C{{H}_{3}}}}\,\] \[\underset{\text{isoamyl}\,\text{alcohol}}{\mathop{HO-C{{H}_{2}}-C{{H}_{2}}-CH-C{{H}_{3}}}}\,\]
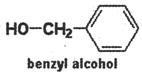
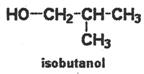
![]() Physical Properties
Physical Properties
They can react to form ester compounds, and they can undergo nucleophilic substitution reactions. The lone pairs of electrons on the oxygen of the hydroxyl group also makes alcohols nucleophiles.
![]() Applications
Applications
![]() Production
Production
In industry, alcohols are produced in several ways:
![]() Etherification
Etherification
To form an ester from an alcohol and a carboxylic acid the reaction, known as esterification, is usually performed at reflux with a catalyst of concentrated sulpuric acid:
\[R-OH+R'-COOH\to R'-COOR+{{H}_{2}}O\]
In order to drive the equilibrium to the right and produce a good yield of ester, water is usually removed, by an excess of \[{{H}_{2}}S{{O}_{4}}\]. Esters may also be prepared by reaction of the alcohol with an acid chloride in the presence of a base such as pyridine.
![]() Oxidation
Oxidation
Primary alcohols \[(R-C{{H}_{2}}-OH)\] can be oxidized either to aldehydes \[(R-CHO)\] or to carboxylic acids \[(R-C{{O}_{2}}H)\] while the oxidation of secondary alcohols \[({{R}_{1}}{{R}_{1}}CH-OH)\] normally terminates at the ketone \[({{R}_{1}}{{R}_{2}}C=O)\] stage.
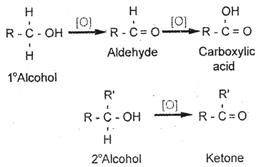
Tertiary alcohols \[({{R}_{1}}{{R}_{2}}{{R}_{3}}C-OH)\] are resistant to oxidation. The direct oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde.
It is then transformed via an aldehyde hydrate \[(R-CH{{(OH)}_{2}})\] by reaction with water before, it can be further oxidized to the carboxylic acid.
![]() Carboxylic Acids
Carboxylic Acids
The organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is \[R-COOH,\] where R is some monovalent functional group. A carboxyl group is a functional group consisting of a carbonyl \[(RR'C=O)\] and a hydroxyl \[(R-O-H)\] which has the formula, usually written as \[-COOH\] or -\[C{{O}_{2}}H\].
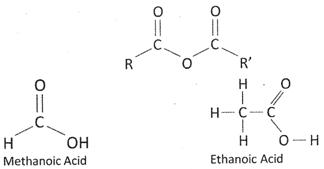
Other important natural examples are citric acid in lemons and tartaric acid in tamarinds.
Salts and esters of carboxylic acids are called carboxylates. Carboxylic acids can be seen as reduced or alkylated forms of the Lewis acid carbon dioxide. Under some circumstances they can be decarboxylated to yield carbon dioxide
![]() Physical properties
Physical properties
![]() Ethanoic Acid
Ethanoic Acid
Acetic acid is an organic compound with the chemical formula \[C{{H}_{3}}COOH\]. It is a colourless liquid, that when undiluted is also called glacial acetic acid. As the main component of vinegar, it has a distinctive sour taste and pungent smell. Although it is classified as a weak acid, acetic acid is highly dangerous to skin.
![]() Chemical Properties
Chemical Properties
![]() Reaction of Acetic Acid with Methanol
Reaction of Acetic Acid with Methanol
Acetic acid reacts with methanol in presence of acid catalyst to gives easter.
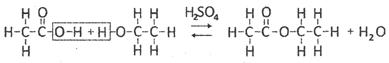
Ethanoid acid Ethanol Ethyl ethanoate
![]() Reaction of Acetic Acid Bose
Reaction of Acetic Acid Bose
Ethanoic acid reacts with sodium hydrogen carbonate to given sodium acetate with evolution of carbon dioxide and water.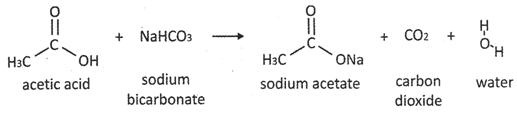
![]() Occurrence and Applications
Occurrence and Applications
![]() Synthesis
Synthesis
.
You need to login to perform this action.
You will be redirected in
3 sec
