Carbon and Its Compounds
Category : 10th Class
Carbon and Its Compounds
We all are familiar with the black amorphous forms of carbon which are coal, charcoal and soot. Most of the things such as food, medicines, clothes, paper contain carbon. Carbon is the 6th element in the periodic table. All living organisms contain carbon. Carbon is also present in small amount in the earth's crust and in the atmosphere.
Carbon is a tetravalent element. It can occur either in free state as diamonds, graphite and buckminster fullerene or in the combined state such as carbon dioxide, carbonates, coal, petroleum and organic compounds like carbohydrates, fats and proteins, etc.
Carbon has large number of organic compounds as it can form long chains of its own atoms. This is a unique characteristic that carbon element have among all other elements. This property to form long chains of its own atoms is called catenation. Important structural elements of life are formed from long chains of carbon atoms that exist in different molecular forms. And another reason for the large number of organic compounds of carbon is that the valency of carbon is 4 (which is quite large).
Hydrocarbons
Carbon forms large number of compounds with hydrogen element called hydrocarbons. The hydrocarbons are categorized into two categories, namely saturated hydrocarbons and unsaturated hydrocarbons.
Saturated Hydrocarbons
Saturated hydrocarbons are the compounds of carbon and hydrogen in which carbon atoms contain only one carbon-carbon bond. The bond between carbon and hydrogen is also single covalent bond. They are called saturated compounds because all the four bonds of carbon are fully utilised and no more hydrogen or other atoms can attach to it. Saturated hydrocarbons can undergo only substitution reactions. Saturated hydrocarbons are also called alkanes. The general formula of saturated hydrocarbons or alkanes is \[{{C}_{n}}{{H}_{2n+2}}\] where n is the number of carbon atoms.
Unsaturated Hydrocarbons
Unsaturated hydrocarbons are the compounds of carbon and hydrogen that contain one double covalent bond between carbon atoms or a triple covalent bond between carbon atoms. In these compounds all the bonds of carbon are not fully utilized by hydrogen atoms and thus more hydrogen atoms can be attached. These compounds can undergo addition reactions to add hydrogen because they have two or more hydrogen atoms less than the saturated hydrocarbons or alkanes \[({{C}_{n}}{{H}_{2n+2}})\]
Unsaturated hydrocarbons containing double bond are called alkenes \[({{C}_{n}}{{H}_{2n}})\]and those that contain triple bond are called alkynes\[({{C}_{n}}{{H}_{2n}}_{-2})\].
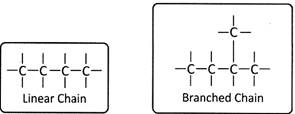
The carbon atom chain can be a linear or branched chain which is an open chain.
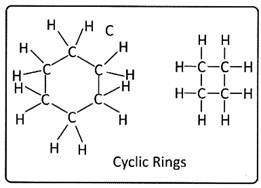
The carbon atom chain can be cyclic or closed rings, sheets and even three-dimensional lattices.
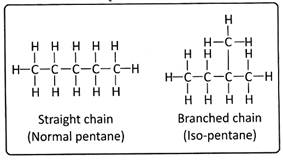
When there are more than three carbon atoms, compounds can be branched. The branched chains provide different structure to the parent alkane and are named differently. For example, in pentane \[({{C}_{5}}{{H}_{12}})\] there exist a straight chain and branched chain called Iso-pentane. Carbon can thus form large number of compounds that have different chain length and structure and are formed by combining with different elements.
Isomerism
The carbon compounds can have same molecular formula, but different structures, which gives different properties to the compound. This phenomenon of different structural formula of me same molecule, giving rise to different properties of compounds, is called Isomerism. The compound with the same molecular formula but a different arrangement of atoms in the molecule are called isomers.
Affinity of Carbon with Other Elements
When an atom or a group of atoms form a bond with the carbon atom in the chain or ring of an organic compound, while showing some characteristic properties of their own, they are termed as a functional group. These functional groups confer specific properties to the compound, regardless of the length and nature of the carbon chain. Thus a functional group is the site of chemical reaction in an organic compound and all compounds containing a particular functional group undergo similar reactions.
Homologous Series
In chemistry, a homologous series is a series of organic compounds with a similar general formula, possessing similar chemical properties due to the presence of the same functional group and shows a gradation in physical properties as a result of increase in molecular size and mass. For example, ethane has a higher boiling point than methane since it has more intermolecular forces with neighboring molecules. This is due to the increase in the number of atoms making up the molecule. Organic compounds in the same homologous series vary by a\[C{{H}_{2}}\]group.
Nomenclature of Carbon Compounds
The names of compounds in a homologous series are based on the name of the basic carbon chain modified by a prefix phrase before or suffix phrase after that indicates the nature of the functional group.
Following method can be used for naming a carbon compound:
Combustion of Carbon Compounds
Fuels are usually hydrocarbons. Combustion of a fuel is an exothermic chemical reaction. Combustion of a fuel involves chemical reaction between fuel and oxidant that results in the production of heat or both heat and light. In a complete combustion reaction, a compound reacts with an oxidizing element, such as oxygen or fluorine and the products are compounds of each element in the fuel with the oxidizing element.
Oxidation
Carbon compounds can be easily oxidised on combustion. In addition to this complete oxidation, we have reactions in which alcohols are converted to carboxylic acids.
Some substances are capable of adding oxygen to others. These substances are known as oxidizing agents. Alkaline potassium permanganate or acidified potassium dichromate oxidises alcohols to acids and thus acts as oxidising agents.
Addition Reaction
Saturated hydrocarbons can be obtained when hydrogen is added to unsaturated hydrocarbon in the presence of catalysts such as palladium or nickel. This reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst to obtain vegetable ghee. Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains.
Oils containing unsaturated fatty acids are considered healthy as they get digested easily, and should be used for cooking. Animal fats generally contain saturated fatty acids which are said to be harmful for health.
Substitution Reaction
Saturated hydrocarbons are fairly unreactive. However, in the presence of sunlight, chlorine is added to hydrocarbons in a very fast reaction. Chlorine can replace the hydrogen atoms one by one. This type of reaction is called substitution reaction because one type of atom or a group of atoms takes the place of another. A number of products are usually formed with the higher homologous of alkanes.
You need to login to perform this action.
You will be redirected in
3 sec
